 The
Science Notebook
The
Science NotebookGilbert Atomic Energy - Part III
Home Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!

 The
Science Notebook
The
Science NotebookHome Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!

We have described several ways in which alpha, beta, and gamma rays may be detected. We have used the Geiger Counter, the Electroscope, and the Cloud Chamber to make various measurements of the range of the rays and their penetrating power. Now we want to look at these rays in a little different manner. We want to see how they affect photographic film. Later on in the section you will discover how they affect certain crystals. We shall now describe some experiments which involve the use of photographic film We shall mention something about procuring and processing of this film. The sensitivity of ordinary film is very low for beta and gamma rays and if you try to carry out experiments with such film you will find that it takes a very long time for exposure.
Your dentist, however, regularly uses x-ray film in his work. Perhaps you have had an x-ray taken of a tooth and noted that you had to hold a small white rectangle of cardboard behind the tooth. This package is called a dental packet and it contains a small piece of x-ray film 1-1/4 x 1-3/4 inches in size. It is protected against exposure to light by being sealed in the paper container.
Visit your dentist (going to the dentist on your own volition may start a new trend in the history of dentistry !) and ask him for a few packets. Packets may also be obtained from your medical supply house. The A. C. Gilbert Company could not supply film with the Atomic Energy Lab because continuous exposure to the radioactive sources would fog the film.
The technique of film development is very simple and is especially easy for the experiments with radioactivity. If you are unfamiliar with the technique, you are urged to purchase the few items required for developing film and to try your hand at the work. But if you prefer not to do this you can have your film developed for you. Your local photographer will develop the film for you or you can ask your dentist to have them developed for you. By far the more instructive procedure, however, is to do the work yourself.
Take your gamma source and place under it a thick lead washer (such as plumbers use) or some object which you wish to photograph. The object must be dense and it should not cover the entire surface of your dental packet. Place the dental packet (some packets have a thin lead foil backing next to the film; this side should be placed away from the source) under the washer. Hold the sandwich together with a heavy weight or a vise.
40
It may be well for you to fix the three objects in place by taping them to a small board. You may also take two photographs at once by putting another object (such as a key) and film on the other side, making the radioactive source serve as a filler for a “sandwich.” Now put the apparatus where it will not be disturbed. After 24 hours remove the dental packets and develop them. You should see the shadow of the washer or object imprinted on the negative. 1f you use film which is not of the x-ray type, then it will take considerably longer for your exposure.
In order to determine the best exposure you may perform two more experiments. Instead of a washer use a few small lead sheets or iron sheets, each shorter than the other, so that the rays from the gamma source have to travel through different thicknesses of metal to reach the film. Then you should obtain a gamma ray photograph showing steps across the film which show the transmission through the various layers of metal.
As regards exposure time you can use a series of dental packets, exposing them for various time intervals and noting the blackening which is obtained for each exposure time. For uniform results in comparing these negative blackenings, you should develop all your films at the same time. Otherwise you are liable to produce different degrees of blackening by overdeveloping or underdeveloping the various films.
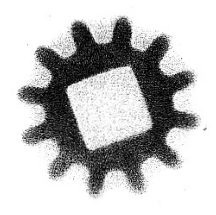
Pictured in Figure 4-1 is a shadow photograph of a key done in much the same way that you did yours. This photo was made by putting a key between a film and some pitchblende ore. You can well see how the emitted particles photograph the key. Figure 4-2 is a sample of what can be done with your own Atomic Energy Lab. This photo was made with the beta source, a gear, and a film.
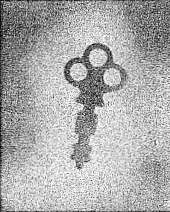
41
Now, it is not easy for you to make a real radioautograph, for it would involve the handling of radioactive materials in a solution which is prohibitive. You can, however, do some experiments with your beta source and at least one can simulate a radioautograph.
As a first, and comparatively crude experiment, take a strip or two of some metal (lead or copper will do), form them in the shape of a cross over your beta source, and then place a dental packet over the cross. Use some tape to fix the “sandwich” firmly in place and allow about 24 hours for exposure. Then develop the film as before and note that the beta rays have been stopped by the lead cross, leaving a clear cross pattern on the darkened film.
Do not be surprised if the film does not become very dark, for it takes about 50,000 beta particles to produce appreciable darkening on even a very tiny section of film. Your beta source is an extended one, being about 20 square cms. in area and, for this reason, it takes a considerable time for enough beta particles to be emitted to darken the film. Repeat the experiment using your gamma ray source and compare your results.
It is possible to make films or plates which are especially
sensitive to beta rays. In effect a radioautograph is simply a
radiograph in which the source of radiation is distributed
throughout. the object and is thus a radioactive “self portrait.”
Consider, for example, the marine life that inhabits the Bikini Lagoon where an atomic bomb was exploded below the surface of the water. Some of the fish feeding on small marine bodies accumulated traces of the radioactive
42
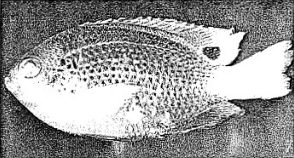

products of the bomb explosion. Figure 4-3 is an actual photograph of a Damsel fish caught in the Bikini lagoon. When this fish was placed on a sheet of photographic film, it took its own (hence the “auto” in the word “radioautograph”) picture which is reproduced in Figure 4-3.
The conclusion to draw is that the Damsel fish concentrated radioactive material in its digestive tract. Smaller white spots on the top fins and tail indicate radioactive particles on the fish.
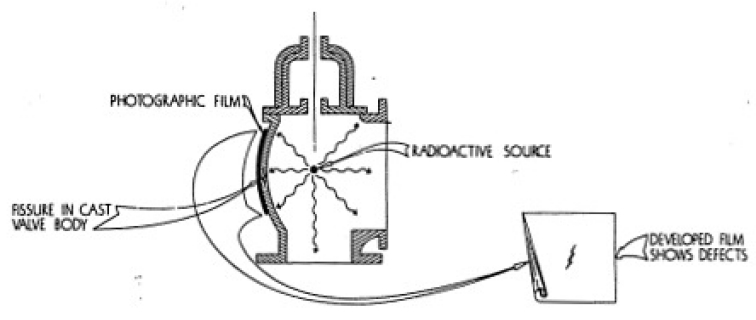
In Figure 4-4 you will see how a radiograph is made commercially. This photograph shows how, by using a radioactive source and a photographic plate, defects in materials can be discovered.
43
We shall now discuss the action radiation has on certain crystals. From the earliest days it was recognized that a screen coated with a layer of special crystals would glow under the action of x-rays. The glow is termed fluorescence or, often, luminescence, and is produced only in certain crystals such as zinc sulfide. Watch dials of the luminous type are coated with a mixture of zinc sulfide and radium.
Alpha particles emitted by the radium activate the tiny crystals and produce a flash of visible light. We can see this by using the Spinthariscope which is provided with the Atomic Energy Lab.
44
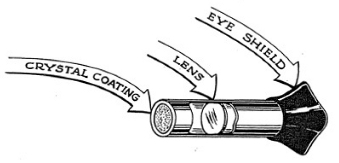
The small tubular instrument,with its rubber eyepiece looks much less sensational than some of the other atomic instruments, but in the history of science it has played an important part. Lord Rutherford, the famous British physicist, carried out his first experiments on atomic structure using fluorescent screens as detectors for alpha particles.
Our Spinthariscope (see Figure 4-5) is a metal tube with a zinc sulfide screen at one end and a lens in the center. You will be able to detect the presence of alpha particles by doing the following experiment.
45
For this experiment it is necessary that your eye be accustomed to almost complete darkness, and therefore the experiment must be carried out in a darkened room. If you simply snap off the light switch and try to do this experiment right away you will not obtain any results, for the eye takes a little time to adjust itself to the darkness.
You have probably noticed when you enter a darkened theater that at first you can see nothing (especially if you come in from bright daylight) and then gradually things begin to take shape and you can see rather well. Perhaps you have also noticed that older people seem to fumble around, unable to see in the darkness, long after the younger people have become accustomed to the lack of light. You will find that it will take a few minutes for your eye to adjust itself, but do not be dismayed if this process takes much longer, especially if you are more advanced in years.
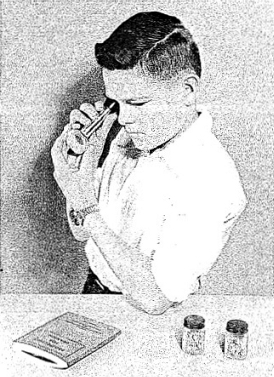
Take your alpha source, place it snugly against the screen end of the Spinthariscope, and then look into the eyepiece. (See Figure 4-6.) If your eye is well adapted to the darkness you should see a number of tiny greenish flashes (scintillations) on the screen. At first it may be difficult for you to see them because of the fact that you are not used to focusing your eyes in the darkness. You should try to focus your eyes on the screen. If you do not see anything, it is possible that your eyes require more adaptation and you should wait for five or ten minutes before trying the experiment again.
To make sure that these tiny flashes are really produced by the alpha particles from the alpha source, you can slip a piece of paper between the source and the screen. The flashes should stop, thus proving that the alpha particles are responsible for the effect.
The Spinthariscope will also detect alpha radiation from your radioactive ores. To do this, merely follow the same procedure as you did in your other experiments, using the Geiger Counter and the Electroscope.
Beta and gamma rays are less efficient in causing flashes in your Spinthariscope.
46
Now that we are familiar with some of the particles emitted by radioactive materials we are going to do a little more exploring. This time you will not be physically working with the instruments of your Atomic Energy Lab in search of the elusive alpha, beta, and gamma ray, but rather you will be exploring briefly the fields of science where these small particles of radioactivity are used.
We shall see what an atom smasher looks like, how a high voltage apparatus works, and the like. And then we will see how atomic energy is put to work in the fields of medicine, agriculture, and industry.
Scientists are people who have an extraordinary zeal for probing into the secrets of nature and puzzling over what they find. What you see here is not the result of one man’s work or one day’s work, but of many men's endeavors over a thousand years. Even these may be the beginnings of other, much better things. But now let us see what has been developed in the science of atomic energy.
Modern scientists, in their search for an understanding of the atom and the nucleus, are leaning heavily on highly technical and complicated apparatus. Much of their apparatus is designed to bombard the atom by artificial means. They study the effect of this bombardment on the atom and by so doing are learning the secrets of the atom itself.
Atomic and nuclear bombardments require some means of obtaining high velocity particles. In general, they are obtained by means of very high voltages or powerful magnets. One of the first such machines was the Van de Graaff Generator.
Professor Van de Graaff at the Massachusetts Institute of Technology devised a very clever means of producing high voltage ‘which has many features that make it an ideal piece of equipment for physical research.
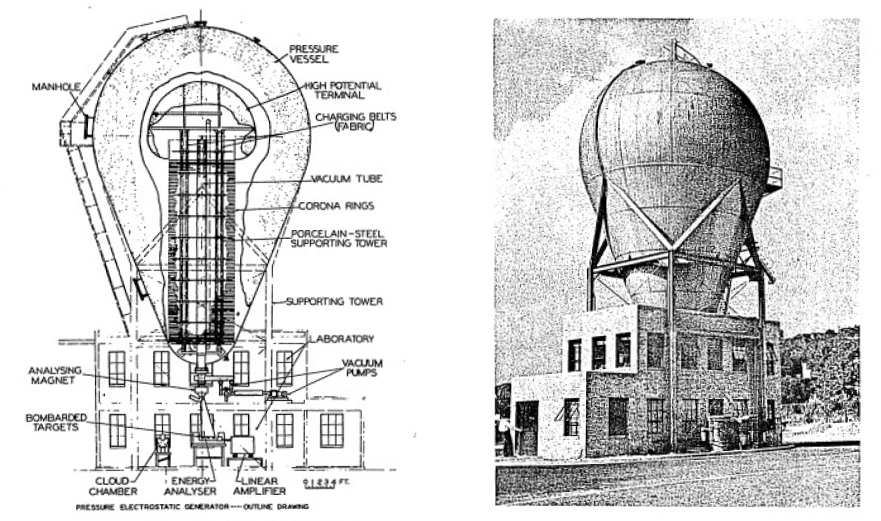
A photograph and a diagram of a Van de Graaff machine is shown in Figures 5-l and 5-2. Impressive as this machine appears, it is not at all complicated in its principle of operation. In fact, it is extremely simple in theory. In a typical Van de Graaff machine a large porcelain (insulating) cylinder is used to support a metal sphere on top.
Inside of this structure is a long endless belt that runs up and down over two pulleys. These pulleys are whirled around by high speed motors and they cause the insulating fabric belt to whirl around them at 70 miles per hour.
47
48
Actually, the belt is nothing more than a conveyor for electric charge. The belt collects a negative charge at the bottom (sprayed on to it by a charge generator) and carries it up to the top where it is picked off by a special set of discharge brushes. The negative charge is distributed over a large sphere while the positive charge goes down the other side of the belt and grounds. In this way large quantities of negative charges collect on the sphere and, as a consequence, the machine charges up to a high voltage.
Machines like the Van de Graaff Generator in conjunction with other apparatus are frequently used to accelerate electrons up to speeds approaching that of the speed of light.
An electron traveling at 99.9 per cent of the speed of light no longer has the same mass as it did when remaining still. Its new weight is 22 times greater than its normal weight. This is no imaginary increase in weight nor is it the dream of some theoretical physicist; it is a very real thing.
The mass increase can actually be measured accurately, and these measurements show that the faster an object goes, the more it weighs. With the ordinary objects of our daily experience, such as a speeding train, the effect is not noticeable, for the velocity of the train represents a minute speed as compared with that of light.
It is only when we invade the realm of atomic particles that we encounter what is called the relativistic mass increase. Professor Einstein’s Theory of Relativity predicts that high speed atomic particles should increase in mass. We shall not attempt to go into this theory; it is enough if we can understand that the mass increase is a real one and one which presents a barrier in accelerating particles to very high energy in a cyclotron.
You may be interested to know that in your television set the electrons which strike the fluorescent screen are about 5% heavier when they bit the screen than when they start out from the heated filament inside the tube. The electrons which are accelerated in a 500,000 volt x-ray tube actually weigh about twice as much when they smash into the tube target as when they start out from the filament.
Figure 5-3 is a photograph of the huge 184 inch super cyclotron, called a synchro-cyclotron, which has been constructed high on a hilltop overlooking San Francisco Bay. The big machine has a yoke type magnet, just as do smaller cyclotrons, and it has enormous magnet coils to supply the magnetic field. The gap between the pole pieces is so large that it is possible to walk between the poles.
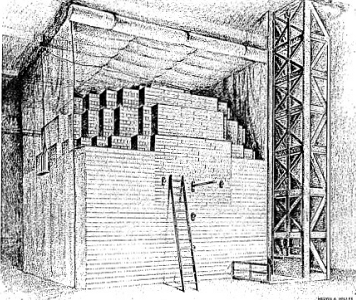
When the machine is running, there is a large amount of penetrating radiation produced and the entire apparatus has to be shielded with concrete to protect personnel in the vicinity. Huge blocks of concrete are used for this purpose.
49
On a large scale the explosion of the U235 atomic bomb is most sensational; yet on a very small scale the explosion of minute amounts of radioisotopes is just as sensational to scientists.
When a radioisotope) such as radiophosphorus, disintegrates and emits radiation, it provides the physicist, chemist, biologist, or technician with a most powerful tool for tracing the secrets of nature. it is in the life sciences that radioisotopes have found most application.
Consider as an example the tracing of a sample of sodium chloride injected into the human body. By tagging the NaCl molecule with radiosodium (radiosodium has a half life of 14.8 hours and emits both beta and gamma rays), and by measuring the radiation emitted from the radiosodium, one has a means of tracing the sodium chloride throughout the body.
50
Physiologists have found that salt thus injected into the body goes into the veins, into the body cells, and is converted into sweat by the body’s sweat glands. It appears on the surface of the skin in the astonishing time of less than one minute. Only because of the tagged sodium atoms can one trace such a fast reaction process.
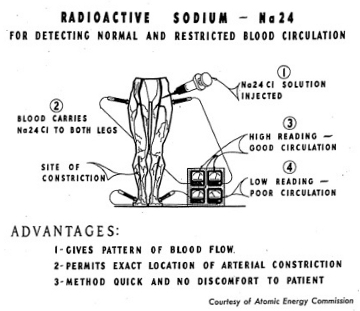
As another example of the use of the atom in the field of medicine, radiosodium is used in tracing the circulation of blond through the body. This is especially useful in cases where circulation is obstructed. Figure 5-4 illustrates graphically the technique used in tracing blood circulation.
The use of radioisotopes is not confined to medicine. In industry radioisotopes are used not only as a means of studying an industrial process, but they are often used to control this process. Many studies on the structure, corrosion, alloying, and friction of metals have been carried out. For example, radioiron produced in a piston ring has been used for friction analysis of a moving piston. By using the easily absorbed radiation emitted from
51
beta active isotopes, thicknesses of films and sheets can be measured without touching the material.
The steel industry has found use for radioisotopes in the study of process control of pig iron. In the printing industry, radioisotopes can be used to measure the amount of ink applied to paper.
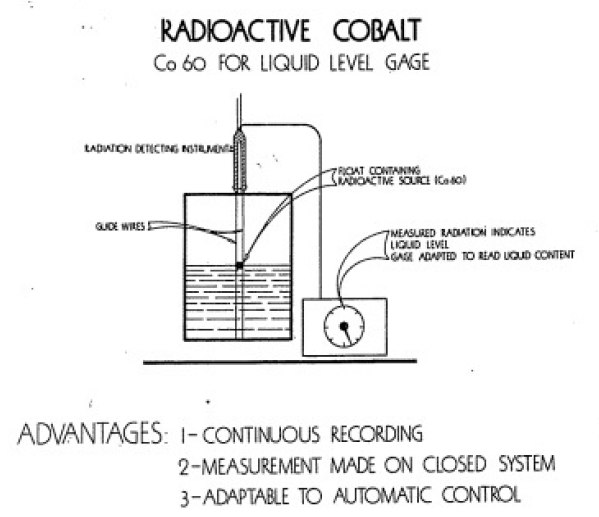
Petroleum scientists have also found extensive use for radioisotopes. Using radiocarbon, the Shell Oil Company and the California Research Corporation have been able to follow changes in oil as it goes through a modern refiner. Sealed sources of radioactive materials are used in determining the level of liquids inside windowless containers (Figure 5-5). Technicians have even been able to determine the rate o! flow of certain liquids through metal pipes by using radiation techniques.
Radioisotopes are becoming increasingly important in agricultural research and in food processing. For example, extensive studies have been made on the efficiency of fertilizers as a source of plant food. Each year American farmers spend over one half billion dollars on fertilizers, so it is extremely important to know how plants utilize fertilizer.
52

One study showed that corn makes maximum use of phosphorus from fertilizer during the early period of growth. Another investigation showed that potatoes use phosphorus throughout the growing season. Still other studies reveal that the manner of applying the fertilizer is often very important. Phosphorus, for example, seems to slow up the growth of potato plants when it is placed very close to seed potatoes.
The Department of Agriculture is very interested in learning how plant diseases and pests develop and attack crops. In this connection, radioisotopes are being used in tagged fungicides, such as sulfur and DDT, to determine how these compounds act.
At the same time, investigations are being carried out with tracers to learn how milk, eggs, and meat can be produced most efficiently. Government experimental stations working with university research units are making considerable headway in these researches.
Still another line of investigation in the realm of plant studies lies in exposing plants to the direct rays of radioactive sources. Workers at the Brookhaven National Laboratory have mounted an intense radiocobalt source in a field (see Figure 5-8) and planted crops at varying distances from the source. The radiocobalt is placed on a pole at the center of the field and the plants are arranged in regular circular patterns. In this way some plants close to the source receive large quantities of radiation whereas those farther away receive proportionately smaller amounts. Plants can then be studied for radiation effect by comparison with those at the edge of the field which receive a very few gamma rays.
53
Today highly significant advances are being made in our understanding of the fundamental physics of nuclear particles. For the most part what we know as basic research was neglected during war. Prior to the war the pump of science had been primed sufficiently full of fundamental facts so that the development and engineering could be emphasized. The end products such as the atom bomb, the proximity fuse, and radar all had their origin in basic knowledge acquired prior to the war.
As a result of this fundamental background, physicists, chemists, and engineers had a fairly good basis on which to proceed. However, the tempo of wartime research and development became so great that often scientists were compelled to go off in the dark for the lack of fundamental knowledge.
54
We may expect that the first practical nuclear power plants will be used as stationary units to demonstrate the practicability of this new power source. Both the Navy and the Air Force will put in high priority requests for power plants which can be used for propulsion. The U.S. Navy has already contracted with the Westinghouse Corporation and is said to have completed the initial design of a nuclear reactor for powering a naval vessel, it will prob ably be from 2 to 5 years before the naval propulsion pile is unveiled.
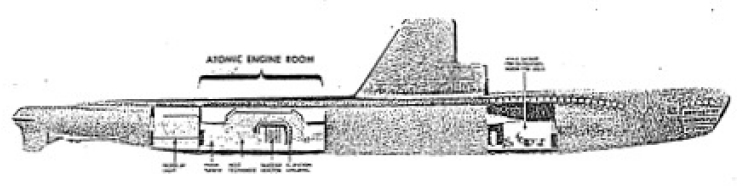
If a reliable propulsion unit is developed we may expect that the Navy will want to use piles for powering its undersea fleet. The submarine is very limited by conventional methods of propulsion, but with the nuclear reactor as a source of power, a true submarine could be built.
By a true submarine, we mean one which can stay submerged almost indefinitely and speed along undersea. Such a submarine would have a truly great advantage over one powered by diesel-electric units. A modern submarine with a 25, 000 kilowatt power pile as the prime source of power could cruise for months without having to refuel, and it would possess the high underwater speed which is necessary for successful battle tactics.
A 25,000 kilowatt power pile would not be excessively heavy and could be installed in a submarine without undue trouble. Figure 5-8 is a model of an artist’s conception of a nuclear-powered submarine.
In the field of nuclear power for the production of electricity, great strides are being made. It will, however, be perhaps several years before nuclear fuel will compete with coal. Furthermore, since the major cost to the consumer of electricity is the cost of transmission of the electricity from the power plant to the consumer, no great saving will result even if a nuclear power plant is feasible.
Estimates of what may happen in the future of science can often be prefaced with the apology that the “Unexpected Usually Happens.” Too often the layman is led to believe that a scientist provided with sufficient money can produce anything that is desired. People point to the Manhattan Project as an
55
example and think that the expenditure of two billion dollars produced the atomic bomb.
Money and concentrated effort may help in a development-engineering project but it cannot automatically remove the physical limits imposed by nature. For example, Einstein’s equation E = MC2 shows that the amount of energy in a U235 atom is 230 billion electron volts of energy. This is more than a thousand times the energy released in U235 fission. Yet this thousand-fold more abundant energy cannot be tapped no matter how many billions of dollars we spend.
Scientists can accomplish what to the layman may seem to be miracles, but they are forever bound to obey the laws of nature. Thus, in making predictions of what to expect in the future, it is well to remember that nature is a stern taskmaster.
Science is not a magic black box from which one conjures up miracles at will. Rather we should say that science is a treasure chest which overflows with the basic ingredients for developing things of use to mankind. The basic ingredients are the hard-won discoveries of physicists, chemists, and the life scientists. Provided with basic knowledge, other scientists and technologists can apply themselves to the task of connecting ideas into practical tools for man’s benefit. Science is such an unpredictable thing that one never knows just where the greatest benefits will arise.
Today we are witnessing a flood of beneficial contributions from science and technology, and so great is the flood that we are at a loss to single out any one field of science as the source of these benefits. Just as a flood arises from the concurrence of many small streams, so, too, does the present abundance of wonder drugs, radioisotopes, jet aircraft, and a million other developments result from the individual contributions of many diverse fields of science.
An electron microscope developed as a scientific tool is found to be essential in isolating a biological virus. Radar developed for wartime application is adapted to marine navigation. The list of such examples is too long to detail but it does illustrate the magnitude of the peacetime benefits which are possible for scientific developments.
Working, as you have, with radioactive sources and with the instruments for measuring their radiations, you have been introduced to but one field of science. It is an exciting and a basic field of endeavor and its off-shoots enter into almost every other field of science.
From our present outlook, it would seem that there will be no single atomic development which will constitute the greatest benefit to mankind. Rather the totality of the many aspects of atomic energy will be most significant. It is in the life sciences that the stimulus of atomic energy will probably have its greatest impact. Here we may expect that the modern tools and techniques of atomic science will contribute substantially to biological and medical research. With these sharpened tools the attack upon the diseases of man, upon cancer, infantile paralysis, tuberculosis, and other dread afflictions will intensify and succeed. If the benefits of modern science and technology are used constructively, man will have within his reach the attainment of a truly more abundant life.
56
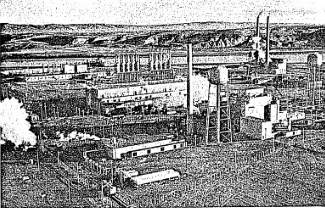
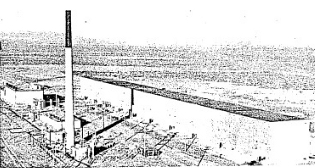
57
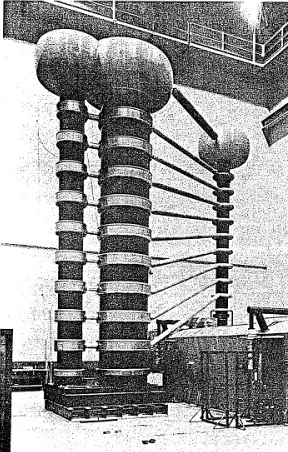
58
The radioactive sources contained ¡n your Gilbert Atomic Energy Lab will not last indefinitely and will, in a period ranging from 1 to 50 years, need replacement.
The life of the Alpha source is approximately 50 years, while the Beta and Gamma sources will remain radioactive for about 2-3 years. The source for the Gilbert Cloud Chamber will deteriorate in about 1 year.
When replacement is needed for these sources, please write to The A. C. Gilbert Company, New Haven 6, Connecticut and state where and when you purchased the set and which radioactive source or sources are required. You will then be informed regarding shipment, cost, etc.
Defective or damaged instruments should be returned to The A. C. Gilbert Company for repair. An estimate will be provided before repairs are undertaken,
59
THE
AC. GILBERT COMPANY
NEW HAVEN 6, CONN., U.S.A.
Printed
in the USA. Copyright 1950 by
The A. C. Gilbert Company
Form
M2788