The
Science Notebook
The
Science NotebookGilbert Chemistry - Part 4
 The
Science Notebook
The
Science NotebookHome Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!
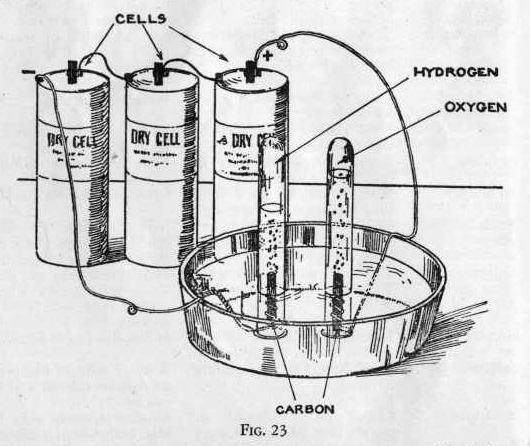
| Gas Air Hydrogen Helium Nitrogen Oxygen |
Specific gravity at 0° C and 760 mm.
1.000
0.06952 0.13804 0.96724 1.10527 |
| |
Hard Water
|
Soft Water
|
| With soap | Forms soap curds, and makes cleaning difficult. | Produces luxurious lather easily. |
| In bathing | Makes skin drawn, clogs the pores, preventing proper elimination of poisons by the skin. | Keeps skin soft and clean. Keeps pores free. |
| In shampooing |
Deposits soap curds in hair, dulling lustre. | Makes hair clean and
glistening. |
| In beauty culture | Makes it impossible to thoroughly cleanse skin. | Keeps skin glowingly clean and free from the blemishes. |
| In shaving | Makes a water-proof coating of soap curds around each whisker, causes razor blade to be dulled. | Thoroughly softens the toughest beard. Makes shaving easy - makes blades last longer. |
| In washing dishes | Causes streaks on china and glassware. | Dishes can just be drained clean. Many pieces do not need to be touched by a towel. |
| In removing grease | Makes work difficult. | Cuts grease like magic. |
| In home laundering | Wastes soap, discolors clothes, often necessitates extra hard washing. | Saves 50-80% of soap formerly used. Makes clothes soft, sweet, clean. Prolongs their life. |
| In washing wood-work, porcelain, etc. | Calls for hard scouring - harms finish. | Preserves lustre. |
| In pipes | Scale chokes down flow of water. Makes faucets drip. | Keeps pipes free from scales. Faucets seat tightly. |
| In water heater | Deposits rock-like scale in coil. Fuel is wasted. | Coils last longer. Minimum of fuel is consumed. |
| In steam boilers | Wastes fuel. Needs a long time for steam to come up. | Saves fuel. Steams quickly. |
| In automobile radiators | Will eventually clog tubes, causing overheating, boiling away of water. | Keeps radiator in efficient condition. Keeps motor operating at proper temperature. |
| In making pie crust | Tends to make crust tough. | Makes crust flaky and tender. |
| In cooking green vegetables | Toughens them, causes loss of flavor. | Keeps them tender with all natural freshness. Need less cooking. |
| In making tea and coffee | Impairs flavor. | Makes them taste better. |
| For drinking | Hard water is unpleasantly flavored. | A water softener like permutet removes calcium and magnesium. |
| For care of baby | Causes rashes blamed on prickly heat. Milk bottles get scummy - harder to clean. | Eliminates many skin troubles. Makes bottles glisten. |
| For hygienic cleanliness | Makes it hard to remove dirt and foreign matter. Prevents thorough cleaning. | Cleans thoroughly and easily. Insures absolute cleanliness. |