The
Science Notebook
Gilbert Chemistry - Part 6
The
Science Notebook
Gilbert Chemistry - Part 6
NOTE: This book was published in 1936 as a manual
to accompany several Gilbert Chemistry sets of the time.
While some of the experiments and activities here
may be safely done as written, a number of them use chemicals
and methods no longer considered safe. In
addition, much of the information contained in this book about
chemistry and other subjects is outdated and inaccurate.
Therefore, this book is probably best appreciated for
its historical value rather than as a source for current
information and good experiments. If you try
anything here, please understand that you do so at
your own risk. See our Terms of Use.
Pages 101- 120
GILBERT CHEMISTRY 101
EXPERIMENT 226 - Pharaoh's serpents
Mix two parts potassium dichromate, (this can be secured in a drug
store), one part of potassium nitrate and one part of sugar. The
ingredients must be powdered separately and then mixed. Small paper
cones can be made and filled with the compound which has been
moistened either with alcohol or water. When dry light at the
top. As the cone burns "snakes" will form.
EXPERIMENT 227 - How to make snakes
These snakes are sold around the Fourth of July as fireworks. They
are made very easily by mixing up four Gilbert measures of sugar,
two measures of sulphur and two of cobalt chloride. Place in a spoon
and heat over a candle. The mass will melt and then it will swell up
in size. It is many times the size of the original mass. By mixing
with alcohol and making into moulds and then allowing to dry you
will be able make quite a stable compound.
EXPERIMENT 228 - Red flame from
sawdust
Boil some sawdust or woodshavings in a cup of water containing a
teaspoonful of potassium nitrate. When 1t is dry it will burn
with a white-yellow flame, sizzling as it burns. Add some
strontium nitrate to the potassium nitrate solution and it will burn
with a red flame.
EXPERIMENT 229 - An aurora borealis
For this phenomenal experiment prepare a mixture of the following
substances on an old tin pan: one measure of strontium nitrate, two
measures of potassium nitrate, one measure of powdered charcoal, one
measure of sulphur, one measure of powdered zinc, one measure of
powdered magnesium and one measure of powdered iron. Do not
rub or grind the mixture. Set the pan in some place where sparks
from the mixture will not damage anything. Now ignite the
mixture with a match or fuse and notice that the mixture will burn
with different colored lights and at the same time produce showers
of bright sparks.
The sparks are caused by the oxidation of the different metals in
the mixture by potassium nitrate.
Obtain a soda water straw and, closing one end by folding it over,
fill it with a mixture of the above substances. Place the
straw containing the mixture in a test tube and going out doors into
the open, light the open end of the straw. Notice the beautiful
effect produced by the burning mixture.
COLOR
IN FIREWORKS
The bright reds, the greens and other colors produced in fireworks
on the Fourth of July depend on the presence of the salt of a metal
which has the property of imparting a particular color to a flame.
In order to impart color, the salt must be volatile, in order for it
to serve in this way. The beautiful red of Roman candles, sky
rockets, are produced by salts of lithium or salts of strontium.
Barium compounds are among the most common ingredients producing
green effects in fireworks. Fireworks also serve very practical
purposes outside the celebration of the Fourth of July. On railroad
trains, for instance, a device known as the "fusee" is carried. This
is a red flare which the brakeman places at the rear of a train that
is stalled, or is used to warn the engineer of an approaching
train. In shipping, aviation, in military activities, rockets
and flares are used for signals.
102
GILBERT CHEMISTRY
ALUMINUM,
ZINC, MAGNESIUM
These are three very important metals and find wide application in
industry.
Aluminum is a very important metal because it is very malleable, is
easily cast, is tenacious, and more rigid than the same weight of
other metals. Its uses are many. It is used for common
household aluminum ware. It is used extensively in the manufacture
of airplanes on account of its lightness and durability. Recently it
has been used to construct the gondolas of the stratosphere
balloons. Aluminum is also used as a conductor in electric power
lines.
Aluminum forms valuable alloys with steel or magnesium.
The compounds of aluminum are important. The silicates are used
extensively in manufacture of cement, brick, tile, earthenware,
pottery and porcelain ware. Several valuable gems contain
aluminum. The ruby, sapphire and topaz are transparent crystals of
aluminum oxide, containing small amounts of certain metallic oxide
which impart the color.
The alums, which are double salts containing aluminum sulphate, are
used as mordants in dyeing and printing. Because of its acid
reaction in water, alum is used in some baking powders.
EXPERIMENT 230 - Colored aluminum
lake
Put two measures of cochineal in a test tube half full of water,
boil over the flame. Now add one measure of aluminum sulphate
and shake until it is all dissolved. Dissolve one measure of
sodium carbonate in another test tube 1/3 full of water and add to
the first solution. Notice the red precipitate. This is aluminum
hydroxide carrying with it the red cochineal. This process is used
in dyeing and in clarifying water.
EXPERIMENT 231 - Action of sodium
carbonate on aluminum sulphate
Dissolve one measure of aluminum sulphate in a test tube 1/3 full
of water. In another test tube 1/3 full of water add one
measure of sodium carbonate and shake until dissolved. Now mix
the two solutions and notice the formation of a gelatinous
precipitate. This precipitate is aluminum hydroxide. Aluminum
carbonate has has never been prepared, because it is a salt of a
very weak acid and a weak base. Consequently it immediately
hydrolyzes completely to form aluminum hydroxide, carbon dioxide and
water. The gas given off in the reaction is carbon dioxide
gas.
Alum baking powder or cream of tartar substitute, as it sometimes
comes on the market, consists of sodium bicarbonate, starch and
potassium alum. The starch is used to keep the materials dry.
Zinc is used in batteries and as a coating for other metals to,
protect them from the oxygen of the air. Galvanized iron is made by
dipping iron into molten zinc and allowing to cool. Zinc forms
several important alloys.
Zinc dissolves readily in both acids and alkalis producing hydrogen
and a zinc salt. Zinc also combines readily with certain other
elements as oxygen and sulphur.
EXPERIMENT 232 - Action of zine on
alkalis
To a test tube half full of sodium hydroxide, prepared as directed
in experiment 202, add one measure of powdered zinc. Warm, if
necessary, to start the reaction. Notice the gas bubbling off
. This is hydrogen.
Magnesium occurs abundantly in nature as the carbonate.
Metallic magnesium is very important commercially because of
its extreme lightness and strength. It is now a rival of
aluminum in construction demanding a light metal. Many of the
large trucks used for transporting new automobiles are constructed
of a magnesium alloy, "Dow metal," which is being extensively
used.
GILBERT
CHEMISTRY 103
When ignited in air magnesium burns with a brilliant white light.
For this reason the powdered metal, mixed with potassium
chlorate (or potassium nitrate) is used as a flashlight powder in
photography, as well as fireworks and signal flares.
The well-known milk of magnesia is magnesium hydroxide and epsom
salts is magnesium sulphate.
EXPERIMENT 233 - How to make a
white flashlight powder
Mix together on an old pan one measure of powdered magnesium and one
measure of potassium nitrate. Do not rub or grind the mixture.
Now place one measure of sulphur on top of the mixture and carefully
light the sulphur. The sulphur will burn and suddenly the mixture
will flash, giving off a very brilliant light.
Try this same experiment, leaving out the sulphur and using a fuse
made by soaking a piece of string in a strong solution of potassium
nitrate and allowing the string to dry. In setting off
the mixture with a fuse, place the mixture on one end of the fuse
and light the other end with a match.
.
EXPERIMENT 234 - How to make a red
flashlight powder
Repeat experiment 233, using one measure of strontium nitrate in
place of the potassium nitrate. Notice the brilliant red light
produced when the mixture is ignited.
235-How to make a green flashlight
powder
Repeat experiment 233, using the following proportions of
substances: one measure of potassium nitrate, two measures of
powdered magnesium, one measure of boric acid, and one measure of
sulphur. Notice this time that a bright green flash is
produced.
Do not allow any of the above flashlight mixtures to remain in the
air too long, as some of the salts take up water from the air and
therefore will not ignite quickly.
EXPERIMENT 236 - Making
sparklers
Mix together on a sheet of paper one measure of potassium nitrate
and two measures of powdered magnesium. Do not rub or grind
the mixture. Now melt some paraffin in your spoon and dip a match
into the liquid paraffin until it is well coated. Remove the match
from the paraffin and after a few seconds roll it in the mixture of
magnesium and potassium nitrate. When you have a good covering of
the mixture on the match, allow the match to dry thoroughly for
several minutes. Now light the end of the match and notice that it
will burn and give off bright sparks. The 4th of July sparklers are
prepared in a manner similar to this.
THERMITE
EXPERIMENT 237 - Thermite fusion
This well-known experiment on thermite can always be depended upon
to arouse an intense enthusiasm for science. The experiment should
not be undertaken, however, without the advice and direction of an
older person, and should not be conducted in surroundings where
there is danger of fire and injury to property. It is a
perfectly safe experiment to perform, but the necessary chemicals
are not supplied in sufficient quantity in your set, and must be
purchased in the market. For the demonstration, it is first
necessary to prepare a plaster of Paris cone. This is made by
coating the inside of a large funnel with vaseline. A large paper
cone is placed inside, and it is also coated with vaseline.
A hollow plaster of Paris cone is now made, using the prepared
funnel as a mold. Before the plaster sets, a hole is made in
the bottom of the cone. When the plaster is hard, it can
be very easily slipped from the funnel.
104
GILBERT CHEMISTRY
The cone is placed in a large ring on a ringstand, at the base of
which is a pan [?] of sand. A piece of paper is stuffed into
the opening at the bottom of the cone so as to prevent the ignition
mixture from dropping out. The cone is then filled with the mixture
of aluminum powder and iron oxide. Other oxides, of course, may be
substituted. To insure a good yield of molten metal, a liberal
number of iron brads is added to the mixture.
On top of the mixture a very small heap of an oxidizing agent is
placed. A piece of magnesium ribbon is then stuck into the
oxidizer, the cone is covered with a sheet of asbestos, containing a
small hole for the magnesium strip, and the magnesium ribbon is
ignited.
This demonstration is most dazzling and impressive when shown in a
darkened room.
SILICATES
EXPERIMENT 238 - Silicic acid
Put 1/2 inch of water glass in a test tube and add water until the
tube is one-quarter full. Shake to mix the liquids.
In another test tube put four measures of sodium bisulphate and fill
the tube one-third full of water. Shake until the solid is
completely dissolved.
Pour the sodium bisulphate solution into the water glass. A
jelly-like precipitate will form, and in a few minutes all the
liquid in the tube will become solid.
EXPERIMENT 239-Sodium silicate
(water glass)
Paint a thin film of water glass on a sheet of paper and let it dry
for 15 or 20 minutes. Note the smooth transparent glass-like film
which results.
Paste together two sheets of paper or two blocks of wood, using
water glass as the adhesive. You will find that it makes an
exceptionally strong paste and it is often used for this purpose.
EXPERIMENT 240 - Strontium silicate
Dissolve two measures of strontium chloride in half a test tube of
water and add two or three drops of water glass.
A bulky white precipitate will form and upon shaking the test tube
the precipitate will fill the whole tube. `
EXPERIMENT 241 - Zinc silicate
Place a small piece of zinc metal and two measures of sodium
bisulphate in a test tube. Fill the tube half full of water, heat
the solution for a moment and wait until some of the zinc dissolves.
Now hold the tube in a glass of cold water for a moment or two until
it becomes cool.
Add to the solution of zinc sulphate thus formed two or three drops
of water glass. Zinc silicate will be formed.
EXPERIMENT 242 - Aluminum silicate
Place two measures of aluminum sulphate in a test tube and fill the
tube half full of water, shake to completely dissolve the solid.
Now add two or three drops of water glass and note the thick
white precipitate of aluminum silicate which is immediately formed.
EXPERIMENT 243 - Nickel silicate
Place two measures of nickel ammonium sulphate in a test tube and
fill the tube half full of water. Warm the solution for a few
moments to completely dissolve the
GILBERT
CHEMISTRY 105
solid, and immerse the tube in cold water to cool it again.
Now add two or three drops of water glass to the solution of nickel
ammonium sulphate and you will get a beautiful green precipitate of
nickel silicate.
EXPERIMENT 244 - Ferrous silicate
Dissolve two measures of ferrous ammonium sulphate in a test tube
half full of water. To this solution add two or three drops of
water glass and a thick precipitate of ferrous silicate will be
formed.
EXPERIMENT 245 - Ferric silicate
Dissolve two measures of ferric ammonium sulphate in a test tube
half full of water and add two or three drops of water glass. A very
pretty reddish-brown precipitate of ferric silicate will be formed.
EXPERIMENT 246 - Tin silicate
Put one measure of sodium bisulphate, one measure of ammonium
chloride, and a small piece of tin metal into a test tube. Add five
or six drops of water and heat the liquid, allowing it to boil for
two or three minutes. Pour the clear solution into another clean
test tube and add water until the tube is one-quarter full.
Now add two or three drops of water glass and in a few moments a
thick white precipitate of tin silicate will form.
EXPERIMENT 247 - Chromium silicate
Dissolve two measures of chrome alum in a test tube full of water.
Add two or three drops of water glass and a beautiful thick green
precipitate of chromium silicate forms immediately.
EXPERIMENT 248 - Cobalt silicate
Dissolve one measure of cobalt chloride in half a test tube of water
and add two or three drops of water glass.
In this case a beautiful blue precipitate of cobalt silicate is
formed.
EXPERIMENT 249 - Copper silicate
Place two measures of sodium bisulphate and one measure of
copper sulphate in a test tube and fill the tube half full of water.
Heat the liquid for a few moments until a clear blue solution of
copper sulphate is obtained. Add to the copper sulphate solution two
or three drops of water glass and examine the blue precipitate of
copper silicate which is formed.
EXPERIMENT 250 - Manganese silicate
Place two measures of manganese sulphate in a test tube half
full of water and heat the liquid for a few moments to completely
dissolve the solid.
Now add to this solution two or three drops of water glass. A pale
pink precipitate of manganese silicate will be formed.
EXPERIMENT 251 - Magnesium silicate
Dissolve two measures of magnesium sulphate in a test tube half full
of water. Add to this solution two or three drops of water
glass. A white precipitate of magnesium silicate will be formed.
EXPERIMENT 252 - Calcium silicate
Put three measures of calcium chloride in a test tube half full of
water, and shake until the solid is dissolved. Now add two or three
drops of water glass and you will get a white precipitate of calcium
silicate.
106
GILBERT CHEMISTRY
FERROCYANIDES
EXPERIMENT 253 - Zinc ferrocyanide
Dissolve two measures of sodium ferrocyanide in a test tube half
full of water. In another test tube put a small piece of zinc
metal and two measures of sodium bisulphate. Fill the test tube 1/4
full of water and shake until the solids are dissolved. Now
add a few drops of the sodium ferrocyanide solution from the first
test tube and a white precipitate of zinc ferrocyanide will be
formed.
EXPERIMENT 254 - Aluminum
ferrocyanide
Dissolve one measure of aluminum sulphate in a test tube half full
of water. Add a few drops of the solution of sodium
ferrocyanide prepared before and a light brown precipitate of
aluminum ferrocyanide will be formed. If this precipitate appears
blue it shows that there is a trace of iron in the aluminum
sulphate.
EXPERIMENT 255 - Nickel
ferrocyanide
Dissolve one measure of nickel ammonium sulphate in a test tube half
full of water. Add a few drops of the solution of sodium
ferrocyanide prepared before and a light green precipitate of nickel
ferrocyanide will result.
EXPERIMENT 256 - Ferrous
ferrocyanide
Dissolve one measure of ferrous ammonium sulphate in a test tube
half full of water and add to this solution two or three drops of
sodium ferrocyanide solution. The resulting bluish white
precipitate which forms is the same thing as Turnbull's blue.
EXPERIMENT 257 - Ferric
ferrocyanide
Dissolve one measure of ferric ammonium sulphate in a test tube half
full of water and add a few drops of sodium ferrocyanide solution.
The deep blue color which results is called Prussian Blue, which is
ferric ferrocyanide.
EXPERIMENT 258 - Manganese
ferrocyanide
Dissolve one measure of manganese sulphate in a test tube half full
of water. When you add to this solution a few drops of sodium
ferrocyanide solution a white precipitate of manganese ferrocyanide
is formed.
EXPERIMENT 259 - Cobalt
ferrocyanide
Dissolve one measure of cobalt chloride in a test tube half full of
water. Add to this solution a few drops of sodium ferrocyanide
solution and a pretty green precipitate of cobalt ferrocyanide is
formed.
EXPERIMENT 260 - Chromium
ferrocyanide
Dissolve one measure of chrome alum in a test tube 1/4 full of
water. To this add a few drops of sodium ferrocyanide solution and a
deep green color will appear, due to the formation of chromium
ferrocyanide. Notice that in this case the color is not in the form
of a precipitate as the chromium ferrocyanide is soluble.
EXPERIMENT 261 - Tin ferrocyanide
Put one measure of sodium bisulphate, one measure of ammonium
chloride, and a small piece of tin metal in a test tube and add five
or six drops of water. Heat the solution, allowing it to
boil for two or three minutes to dissolve some of the tin. Now
pour the clear solution into another test tube and add a few drops
of the clear solution into another test tube and add a few drops of
sodium ferrocyanide solution. Note the light bluish green
precipitate of tin ferrocyanide which is formed.
[107]
PART III
Organic Chemistry and Its Commercial Application to the
Industries
CARBON
Organic chemistry is based on our knowledge of the properties and
reactions of compounds of the element, carbon. This element is found
in nature in the free condition in several forms. The diamond is
practically pure carbon, while ordinary coal and graphite contain
small percentages of other substances besides carbon as mineral
matter. Naturally occurring compounds of carbon are of wide
occurrence in nature and are found in the form of gases, liquids or
oils and solids. Carbon dioxide is the most familiar gaseous
compound of carbon. Manufactured illuminating gas, natural gases
from wells and petroleum are all composed chiefly of organic
compounds of carbon and hydrogen.
The carbonates, especially calcium carbonate, constitute a very
large proportion of the natural rocks and some form of mineral
carbonate are found in most localities. The building stone -
marble - is a very pure form of calcium carbonate. Carbon
constitutes a large percentage of all living organisms, both plant
and animal, and is represented in such organic products as proteins,
fats, sugars and natural oils. All products of these types are
widely utilized by man as food and for the manufacture of useful
commercial products. At the present time more than 300,000 organic
compounds are known, and the possibilities of new creations as the
science of organic chemistry is developed are unlimited. Of all the
elements occurring in nature, carbon is the one which is most
commonly associated with life itself. Our present world could not
exist without this element.
Coke is a modified form of impure carbon, and is used as a fuel in
operating steam boilers, and also in smelting processes for refining
ores. It is made by heating bituminous, or as it is more commonly
known, soft coal until all volatile products in the coal have been
expelled. This heating process is conducted without excess of air,
and is carried out out on a commercial scale in large ovens. The
volatile or gaseous products are refined and constitute the raw
materials for the manufacture of illuminating gas and low boiling
hydrocarbons like benzene.
Every boy and girl is familiar with ordinary charcoal. This is a
form of carbon produced by the destructive distillation of organic
substances such as wood and sugar, and even bones of animals. By
destructive distillation is meant heating without access to air as
in the manufacture of illuminating gas. The quality of wood charcoal
obtained is dependent on the kind of wood which is subjected to
destructive distillation. It is known, for example, that the
destructive distillation of cocoanut shells is productive of a very
efficient form of absorbent carbon meeting the exacting requirements
of the gas mask. If air was admitted during the distillation
process, the charcoal and gaseous products would be burned up
completely with formation of carbon dioxide gas and water.
Another form of carbon is graphite. This is the black substance
which forms the core of lead pencils. lt is sometimes referred to as
lead, but this is not correct. It is not lead, but a modified form
of carbon, and can be made by subjecting carbon to a very intense
heat. At about 4000į C. carbon vaporizes, and this vapor on
condensing forms graphite. Graphite is used in the manufacture
of crucibles, as a lubricant, as a
108
GILBERT CHEMISTRY
protective covering for metals such as stove polish, and in the
manufacture of lead pencils.
Lamp black, an amorphous form of carbon, finds wide application in
the trades. It is used in rubber as a toughening agent; in
printers' ink as a pigment; in paints, stove polishes and lacquers
as a pigment; on typewriter ribbons and and carbon papers as a black
coloring matter. Bone black is an amorphous carbon produced by
destructive distillation of bones. It is used as a deodorizing and
decolorizing agent.
THE
MANUFACTURE OF ILLUMINATING GAS
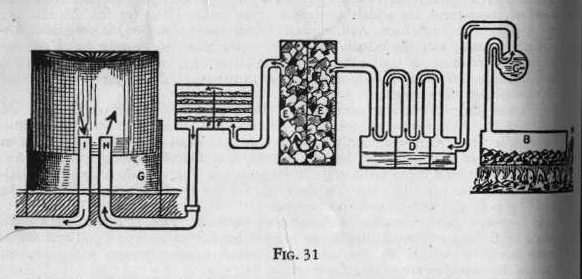
Cylindrical ovens of Fire clay (B-Figure 31) are filled
automatically with soft coal. These ovens are then closed
tightly to prevent entrance of air. Under these ovens is a hot
fire (A). The heat decomposes the coal into gases, liquids and
coke. The gases contain impurities such as hydrogen sulphide,
carbon dioxide, ammonia, tar, benzol, toloul and water. These
impurities are removed before the gases are passed into receiving
tanks.
Then first step towards purification of the gases is as follows: The
gases pass from the oven through a pipe and bubble through running
cold water contained in the lower half of a large pipe (C).
Here coke dust (carbon), tar oils and ammonia are removed.
Second, the gases are then passed through an arrangement (D) which
consists of several hundred feet of pipe. This acts as a condenser
and cools the gas down to ordinary temperature and condenses the
liquid products of the distillation.
Third, the gases pass from the condenser through a "scrubber" where
they are washed and cleaned. The scrubber (EE) is a large iron tank
filled with coke, crushed rock, wood, and scraps of tin, the object
being to expose a large surface to the gas. A spray of water
is introduced at the top of the scrubber and the material filling it
is thus kept moist. The remainder of the tar and ammonia salts are
here removed and the gasses pass on to the purifier.
Fourth, the gases are passed through the "purifier" (FF), a
rectangular box filled with layers of quick lime, which
absorbs water, carbon dioxide and hydrogen sulphide.
After the moisture is removed, the gases, which now consist of
hydrogen, nitrogen, marsh gas, olefiant gas, acetylene and carbon
monoxide are delivered immediately into the large gas tanks (G).
These tanks are constructed in telescopic fashion so
GILBERT
CHEMISTRY 109
that the quantity of gas regulates and controls the size of the
tank. From the tank it is pumped through gas mains to the
homes of the consumers. (H) is the entrance pipe. (I) is the
exit pipe. (K) is the flue or chimney for the fire.
You may have noticed at times the tremendous flame which shoots up
in the vicinity of gas tanks. This is especially noticeable at
night. The cause of this seems to be mysterious. It is
exceedingly simple. When live steam is passed over white hot coke
away from air, in an oven for instance, water gas is formed.
Water gas cannot be used for lighting purposes alone; it is mixed
with the coal gas. Water gas consists of a mixture of hydrogen and
carbon monoxide and is produced by the action of steam on hot
carbon.
After the steam has been passed into the ovens for a time new coke
must be must be added: in other words, the ovens must be charged
regularly. Before emptying the ovens, valves shut off the gas
connection of the oven with the rest of the plant. ln the ovens
there still remains a good deal of gas. This gas must be removed
before the ovens are emptied of the coke. The quickest and best way
is to burn it away. This is done and the gas disappears in a
tremendous flame. Thus the "aurora borealis" of the city is a
mystery no more.
EXPERIMENT 262 - Preparation of
charcoal - pyro-ligneous acid
Break up a few toothpicks or pieces of wood and place them in the
bottom of a test tube. Now put a piece of moistened blue
litmus paper over the mouth of the test tube and heat over an
alcohol lamp or gas flame. Notice that the paper turns red, proving
that an acid is evolved. This acid is called pyro-ligneous acid and
is essentially acetic. Acetic acid is also present in vinegar. Here
it is formed in the process fermentation resulting by the
action of bacteria on sugar.
Now insert the peforated cork with delivery tube, continue heating
and and light the gas that comes off. Notice that it will burn. This
gas is similar to that obtained from the distillation of coal.
When no more gas is evolved, allow the tube to cool, then empty the
contents of the test tube on paper. This is charcoal and is
practically pure carbon.
When green pine (or green cedar wood) is distilled, turpentine and
tar oils are derived from it. The turpentine is the volatile oil,
that is, it passes off as a vapor. The tar oils are the heavy
resinous oils, brown in color, such as you may see at the bottom of
your test tube.
To summarize, when coal is heated without admission of air, coal
gas, ammonia and coal tar are obtained. The is used for lighting and
heating. The ammonia which is derived is purified and finds many
uses. From the coal tar are derived intermediates from which aniline
dyes, disinfectants such as carbolic acid, high explosives and many
other valuable products are manufactured.
It can be seen, therefore, that enormous industries are based upon
this process of heating wood and especially coal without admission
of air.
THE
SMOKING OF HAMS AND MEATS
Pyro-ligneous acid is contained in the smoke of smoke houses where
hams and other foods are cured. ln the pioneer days of New England
agriculture, a smoke house was a common feature of the farmerís
equipment. Usually this was built out of doors, but in many cases
installed in the attic of the farm house. It is due to the
aseptic action of this acid in the smoke that pork and beef products
are preserved. The probable action of the gradual deposition
of pyro-ligneous acid upon a ham is to gradually kill all the
bacteria or germs which are the cause of decay. The acid also
imparts that distinctive taste which is characteristic of smoked
food.
110
GILBERT CHEMISTRY
Meats can be cured over night, while in the smokehouse several days
are required. This quick curing is a rather new method and is
not practiced except experimentally. The ham is painted with
pyro-ligneous acid, which seeps into the meat. It has been
declared that the quick cured ham is as edible as the slow cured ham
and keeps just as well.
EXPERIMENT 263 - Decolorizing
properties of charcoal
Make a solution of potassium permanganate by dissolving a crystal in
a test tube half full of water. Shake until all is dissolved and the
solution is colored purple.
Now put into this solution three measures of powdered charcoal and
closing the mouth of the tube with the thumb, shake vigorously for
two or three minutes. Now filter this solution in the funnel
and notice the color of the liquid that runs through. It is
nearly colorless, showing that charcoal has the property of a
absorbing colors from certain substances.
Repeat this experiment with different colored solutions and notice
which of the colors are absorbed by charcoal.
EXPERIMENT 264 - Deodorizing
properties of charcoal
Prepare some hydrogen sulphide water as shown in a previous
experiment and notice the odor. To this solution add three measures
of powdered charcoal, close the mouth of the test tube with your
thumb and shake the test tube for minutes. Now filter this solution
and smell the liquid which runs through. Notice that the odor
has been removed by the charcoal.
'
EXPERIMENT 265 - Absorbing
properties of charcoal
Dissolve a small piece of quinine pill about the size of a pin head
in a test tube half full of water and taste the solution. Notice
that it is bitter.
Now add three measures of powdered charcoal, close the mouth of the
test tube with your thumb and shake for three or four minutes.
Filter this solution and taste a little of the liquid which runs
through. Notice that the bitter taste is gone.
Charcoal then removes colors, odors and tastes from certain
solutions.
ABSORBENT
CHARCOAL
EXPERIMENT 256-Abeorbing coloring
matter with charcoal
Put about 1/8 measure of cochineal in a test tube 1/4 full of
water. Warm the tube for a few moments until the cochineal
dissolves, forming red solution.
Now put two measures of powdered charcoal into the cochineal
solution and close the mouth of the test tube with your thumb and
shake three or four minutes. Filter the solution after you
have shaken it thoroughly in order to separate the charcoal and you
will find that the color will be a great deal lighter than it was in
the original solution. By repeating this process several times
it will be possible to take practically all of the color out of this
solution.
Try this experiment on logwood solution, or solutions containing
dyes, or on any other colored liquids.
EXPERIMENT 257 - Decolorizing
vinegar
Fill a test tube one-quarter full of vinegar which has a brownish or
yellow color. Add two measures of charcoal and shake with the
solution for four or five minutes. Now separate the charcoal
by filtering, and you will find that the color of the vinegar is
lighter. The vinegar can be made almost colorless by repeating this
several times.
GILBERT
CHEMISTRY 111
EXPERIMENT 268 - Absorbing bitter
taste with charcoal
If you can obtain a small amount of quinine you will find that
charcoal will absorb the bitter taste. Fill a test tube one-quarter
full of water and add a very small amount of quinine, about the size
of an ordinary pinhead. Shake this with the water and taste a drop
of the the solution and notice the bitter taste.
EXPERIMENT 269 - Absorbent charcoal
from ground cocoanut shells
Grind up a piece of dry cocoanut shell and thoroughly bake the
material in a copper oven. This should be thoroughly
carbonized by this treatment. After baking then grind the particles
of dried shell to a powder by rubbing in a mortar. Test the
efficiency of this powder as a decolorizing and absorbing agent.
EXPERIMENT 270 - Absorbent charcoal
from butternut shells
Crack some butternuts and eat the meat of the nuts. Then take the
shells and thoroughly crush them and finally carbonize by heating in
a copper oven. After this baking then grind to a powder in a mortar.
Test the efficiency of this powder as a decolorizing agent and
absorbing agent.
EXPERIMENT 271 - Preparation of
absorbent charcoal from hickory nut shells
Follow the same directions as given for Experiment 270.
EXPERIMENT 272 - Preparation of
absorbent charcoal from white birch wood
Carbonize some small pieces of white birch wood in a copper oven.
After this baking, then grind to a powder in a mortar. Test the
efficiency of this powder as a decolorizing agent and absorbing
agent.
EXPERIMENT 273 - Preparation of
absorbent charcoal from pine wood
Follow same directions as given for Experiment 272.
EXPERIMENT 274 - Preparation of
absorbent charcoal from maple wood
Follow same directions as given for Experiment 272.
EXPERIMENT 275 - Preparation of
absorbent charcoal from chestnut wood
Follow same directions as given for Experiment 272.
EXPERIMENT 276 - Surface tension
and the rubber band
Float a thin rubber band on a dish of water and touch the water
inside the band with a wire or tooth pick which has been dipped into
oil. The band will snap out, forming a circle. Now apply oil to the
water outside of the band and the band will again resume its
original shape.
This experiment illustrates the effect of surface tension which
tends to make liquids assume those forms which expose the least
surface for a given volume.
EXPERIMENT 277 - Changing the
specific gravity of charcoal
Wood charcoal floats in water. Tie a weight on a piece of charcoal
with a thread so that it will sink and place it in a test tube
one-quarter full of water. Boil the water several minutes,
then remove the weight from the charcoal, and you will find that it
no longer floats. This is due to the fact that the air was driven
away from the pores of the charcoal by boiling. This
experiment illustrates why wood becomes waterlogged and does
not float.
112
GILBERT CHEMISTRY
CARBON
DIOXIDE OR CARBONIC ACID GAS
When carbon and any combustible compound of carbon is burned, the
carbon is converted into carbon dioxide as the final product of
oxidation. This is a gas heavier than air, and is the most commonly
known of all carbon derivatives. While it does not support
combustion, it does serve a valuable purpose in both human and plant
economy. In the plant kingdom, carbon dioxide is absorbed from the
air through the cells of the leaves and furnishes the source of
carbon for building up plant tissues. The transformation of carbon
dioxide in the plant is brought about under the agency of the sun's
rays and the influence of the green chlorophyll of the leaves of the
plant.
While carbon monoxide is poisonous, carbon dioxide is a harmless
gas. It is a waste product thrown off by the lungs during
respiration. lt has been proved that carbon dioxide in the lungs is
responsible for stimulating the respiratory center in the process of
breathing. The deep and rapid breathing from extensive violent
physical exercise, like baseball and football, is not due
directly to the need of oxygen, but rather to the need of
eliminating carbon dioxide from the lungs. The increase of the
carbon dioxide production is a measure of the work being done under
violent exercise. Use is made of carbon dioxide for administration
to patients suffering from hiccoughs. Also to increase
the breathing rate after an anethesia and even after carbon monoxide
poisoning. While carbon dioxide is not ordinarily considered
poisonous, it can, however, be responsible for death, because it
will not support combustion. The ordinary procedure of testing
the air in a mine or a deep well, or any building or inclosed place
having poor ventilation, with a lighted candle has proved to be very
wise in many cases.
MEDICAL
USES OF CARBON DIOXIDE
Ordinarily we think of carbon dioxide as being associated with fire
extinguishers, and for this reason it is sometimes hard for most of
us to appreciate that carbon dioxide can play an important part of a
life-saver and alleviator of pain. We now know that carbon dioxide
stimulates respiration and serves as a means of increasing the rate
of breathing. Advantage is taken of this effect in certain
cases where breathing is suspended, as during asphyxiation,
physical shock, and partial drowning. Machines for
administering mixtures of oxygen and carbon dioxide have proved more
capable of giving relief in these types of cases than those which
use oxygen alone. The breath stimulating effect of carbon
dioxide, and the ventilation produced by oxygen has saved many
lives. Carbon dioxide as an aid in the removal or the anesthetic
when it is longer needed, is a general hospital practice.
CARBONATES
OF METALS
The carbonates of many metals are insoluble in water and can be
precipitated from solutions by means of a soluble carbonate such as
sodium carbonate. In the following experiments you will obtain
many colors. Filter off your insoluble precipitates and note
the color of the carbonate salts.
EXPERIMENT 278 - Strontium
carbonate
Dissolve one measure of strontium chloride in a test tube half full
of water. In another test tube one-quarter full of water
dissolve one measure of sodium carbonate. Now add some of the
sodium carbonate solution to the strontium chloride solution and a
heavy white precipitate of strontium carbonate is obtained.
GILBERT
CHEMISTRY 113
EXPERIMENT 279 - Nickel carbonate
Dissolve one measure of nickel ammonium sulphate in a test tube half
full of water. Dissolve one measure of sodium carbonate in
another test tube 1/4 full of water. Now add the sodium
carbonate solution a little at a time to the solution of nickel
ammonium sulphate and a thick light green precipitate of nickel
carbonate will be formed.
EXPERIMENT 280 - Zinc carbonate
Put a small piece of zinc metal in a test tube, add one measure of
sodium bisulphate and fill the tube 1/4 full of water. Warm this
solution and let it stand for three or four minutes so that some of
the zinc metal will dissolve.
Now prepare a solution of one measure of sodium carbonate in a test
tube 1/4 full of water and add some of this solution to the zinc
solution. A white precipitate of zinc carbonate will be formed which
gradually settles to the bottom of the tube.
EXPERIMENT 281 - Aluminum carbonate
Dissolve one measure of sodium carbonate in a test tube 1/4 full of
water. Dissolve one measure of aluminum sulphate in another
test tube 1/4 full of water. Add the sodium carbonate solution
to the aluminum sulphate solution a little at a time. Notice
that at first an effervescence takes place and then a
gelatinous precipitate of basic aluminum carbonate is formed.
EXPERIMENT 282 - Chromium carbonate
Dissolve one measure of chrome alum in a test tube half full of
water. Add a few drops of sodium carbonate solution as before, and
notice that a bluish green precipitate of chromium carbonate forms.
EXPERIMENT 283 - Ferrous carbonate
Dissolve one measure of ferrous ammonium sulphate in a test
tube half full of water. To this solution add a few drops of a
solution of one measure of sodium carbonate in a test tube 1/4 full
of water. A greenish precipitate of ferrous carbonate will
form.
EXPERIMENT 284 - Cobalt carbonate
Repeat the experiment using one measure of cobalt chloride in place
of the ferrous ammonium sulphate.
EXPERIMENT 285 - Calcium carbonate
Put one measure of calcium chloride in a test tube half full of
water. Shake thoroughly and then add a few drops of sodium
carbonate solution prepared as in the preceding experiment. A
white precipitate will be formed which is calcium carbonate.
EXPERIMENT 286 - Copper carbonate
Place one measure of azurite, which can be purchased at your drug
store, and one measure of sodium bisulphate in a test tube. Fill the
tube half full of water and shake for a few moments until the
solution becomes perfectly clear. Now add a few drops of
sodium carbonate solution prepared as before, and a very pretty
precipitate of copper carbonate will be formed.
EXPERIMENT 287 - Manganese
carbonate
Dissolve one measure of manganese sulphate in a test tube half
full of water. Add a few drops of sodium carbonate solution
prepared as before, and you will obtain an while precipitate of
manganese carbonate.
114
GILBERT CHEMISTRY
EXPERIMENT 288 - Magnesium
carbonate
Add one measure of magnesium sulphate to a test tube half full of
water and dissolve by shaking. Now add a few drops of sodium
carbonate solution, as before, and a white precipitate of magnesium
carbonate will be formed.
CARBON
MONOXIDE
While carbon dioxide is a harmless gas, this member of the carbon
family is a violent poison. It is formed when carbon is burned with
a diminished supply of oxygen. The gas burns with a blue flame being
convened into carbon dioxide.
Several thousand people are killed each year by carbon monoxide gas,
which constitutes a small proportion of the gases expelled through
the exhaust pipe of an automobile.
With the return of cold weather, the spectre of carbon monoxide
poisoning haunts every automobile driver. Unless humanity has
exercised an excess of caution, we may expect occasional news items
about persons warming up their motors in closed garages, being
overcome by this insidious gas. Carbon monoxide is a product of
imperfect combustion. When a fuel is burned under perfect
conditions, carbon monoxide is not produced. The products of perfect
combustion are carbon dioxide and water. Ideal combustion conditions
are difficult to realize. Certainly not in the very best of our
automobiles. An analysis of the exhaust gases of an average
automobile shows about 7% of carbon monoxide. ln a certain
experiment a dog was left in the driverís seat of an automobile in a
closed garage, with the engine running slowly. In twenty minutes the
dog was unconscious. Had a man been in the dog's place, the result
would probably have been the same. Carbon monoxide overcomes
its victim with no warning. The first symptom is a severe pain
in the back of the head, but if the concentration is high, the
victim may lose consciousness before he can act on this
warning. This condition may or may not be preceded by such
warnings as headache, dizziness or nausea. Small doses may
have no other effect than to cause severe headache, but a heavy
gassing is a serious matter. Convalescents from carbon
monoxide poisoning should be kept in bed even when they protest that
they are all right. To avoid carbon monoxide poisoning it
needs no more than good ventilation in the garage. Carbon
monoxide is lighter than air, and vanishes immediately through an
open window or door. A doctor should be called immediately for a
person overcome by the gas. An automobile driven by a driver
under the influence of carbon monoxide constitutes a hazard to the
public safety on the highway equal in seriousness to that of another
car equipped with faulty brakes. It has been found that an
automobile, following too closely behind another, particularly in
heavy traffic, may pick up a sufficient quantity of the exhaust gas
from the preceding car to result in a dangerous mixture within the
second automobile, leading to carbon monoxide poisoning.
ETHYL
GAS
Every automobile driver is familiar today with the trade term -
"ethyl gas." This is an organic compound containing lead which
bestows on gasoline the favorable properties characteristic of this
reagent (tetraethyl lead). It is a practical anti-knock substance.
When ethyl gasoline is used, it tones down the explosions in the
cylinders of gas engines, and pushes the cylinder more gently than
in the case when ordinary gasoline is used. Tetraethyl lead is a
dangerous substance, and warnings, therefore, accompany its
use. At every filling station there are warnings posted to the
effect that ethyl gas should not be spilled upon the hands or used
for cleaning purposes. If ordinary precautions are followed,
tetraethyl gasoline presents little danger during use.
GILBERT
CHEMISTRY 115
EXPERIMENT 289 - Preparation of
carbon dioxide - (effervescence)
make a solution of sodium bicarbonate by shaking up a test tube 1/3
full of water containing two measures of sodium bicarbonate. ln
another test tube make a solution of tartaric acid by shaking up a
test tube 1/3 full of water containing two measures of tartaric
acid. Now pour the tartaric acid solution into the solution of
sodium
bicarbonate.
Notice the violent effervescence due to the chemical action and
liberation of carbon dioxide gas. You will notice that it has
no odor. This is the same gas that you see bubbling out of
soda-water.
EXPERIMENT 290 - Vinegar and baking
soda
Obtain some very strong cider vinegar, the stronger the better, and
place about 3 cc. in a test tube. Then drop into the vinegar a
small measure of baking soda. What gas is given off? Test for
it.
EXPERIMENT 291 - Vinegar and oyster
shells
Repeat the above experiment using a piece of oyster shell. Pulverize
the shell in a mortar before adding it to the vinegar. Warm the
solution and test the gas given off.
EXPERIMENT 292 - Vinegar and
painters whiting
Repeat the above experiment using some painter's
whiting. What is the composition of ordinary whiting?
EXPERIMENT 293 - Vinegar and chalk
Repeat the above experiment using some powdered chalk or crayon from
your school room blackboard.
EXPERIMENT 294 - Vinegar and marble
Repeat the above experiment with some pulverized marble.
EXPERIMENT 295 - Vinegar and tooth
powder
Repeat the above experiment with a good quality of dental tooth
powder. What constituent of tooth powder causes the reaction?
EXPERIMENT 296 - Vinegar and old
mortar
Repeat the above experiment using some old mortar removed from the
walls of an old brick building. Note the evolution of carbonic acid
gas.
EXPERIMENT 297 - Vinegar and
Portland cement
Repeat the above experiment using some pure Portland cement.
EXPERIMENT 298 - Carbon dioxide is
heavier than air, and will not burn
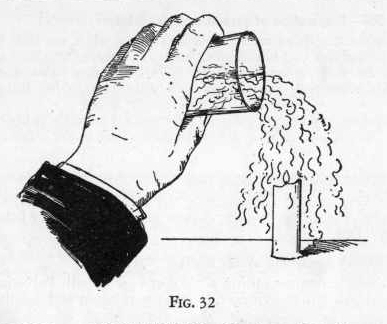
Light a candle and set it firmly on a board by sticking it to a
little melted wax from the flame of the candle. (Figure 32).
Now put one-half teaspoonful of sodium bicarbonate or common baking
soda in a glass and add some vinegar or a solution of tartaric acid
to the glass. A violent reaction takes place with evolution of
carbon dioxide gas.
Now pour the gas in the glass on to the flame just as though you
were pouring water out of the glass, taking care not to spill any of
the acid out of the glass. Notice that the flame goes out,
proving that carbon dioxide is heavier than air and will settle to
the earth and also that it will not burn.
116
GILBERT CHEMISTRY
EXPERIMENT 299 - Chemistry of the
flame
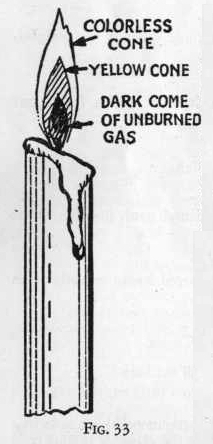
Examine closely an alcohol lamp flame or candle flame and observe
that it consists essentially of three cones. (Figure 53).
First a dark cone just around the wick: second, a yellow cone which
produces! light; and third, a transparent cone of heat around the
outside.
The dark cone of the flame consists of unburned gases which are
given off from the wick of the candle. The paraffin is melted
by the heat and drawn into the wick by capillary action. In
the wick the paraffin is converted a gas by the heat of the flame.
To prove this, hold one end of a hollow glass tube in the
flame just over the wick. Now apply a flame to the other end
of the tube and it takes fire. The gas in this cone is
relatively cool, for if a match stick is placed in it that portion
of the stick which was in the dark cone will not burn as soon as the
portion passing through the sides of the flame.
The second or yellow cone of the flame consists of particles of
carbon that have been heated to white heat so that they glow
brightly.
Hold a cold spoon or glass rod in this cone for a minute and notice
that it is covered with a black deposit of carbon called lampblack,
thus proving that this cone consists of small particles of carbon.
The cold spoon chilled the flame, thereby causing the carbon
particles to be deposited. Lampblack is made upon this principle on
a manufacturing scale.
The third or outer cone of the flame consists of the gases formed by
the complete burning of the carbon particles to carbon dioxide gas.
This is the hottest portion of the flame, and whenever heating a
liquid in a test tube, for example, it is important in order to
obtain the highest heat possible and to prevent the deposit of soot,
to hold the test tube at the tip of the luminous or light-giving
part of the flame.
GILBERT
CHEMISTRY 117
EXPERIMENT 300 - Carbon dioxide
from a burning candle
Make some lime water by putting two measures of calcium oxide
in a test tube half of water and shaking well for three or four
minutes. Allow this solution to stand until clear, then pour the
clear liquid into another test tube. You now have a clear solution
of lime water or calcium hydroxide.
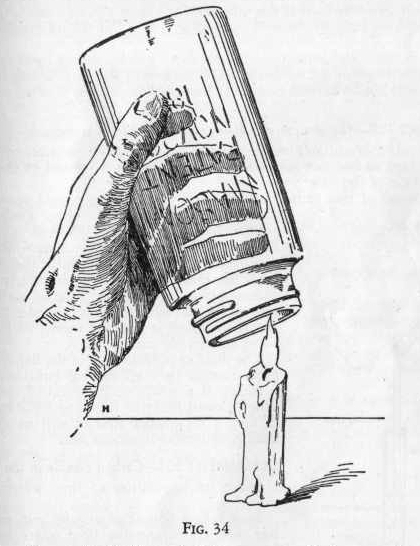
Now hold a wide mouthed bottle or fruit jar over a candle flame as
shown in Figure 34 so that the burning gases from the flame may
enter the mouth of the bottle. After allowing the gases to
enter the bottle for about a minute, close the mouth of the bottle
with the palm of the hand, and, inverting the bottle, pour the lime
water into it. Again put the palm over the mouth of the bottle
and shake for a moment. Notice that the lime water becomes turbid or
milky. This turbidity is due to a white precipitate or calcium
carbonate formed by the action of carbon dioxide on calcium
hydroxide.
EXPERIMENT 301 - The structure of a
flame - A gas factory
If you will look closely at a candle flame you will see that it
consists of three parts.
First, a dark zone just around the wick.
Second, a bright yellow zone which gives the light.
118
GILBERT CHEMISTRY
Third a transparent zone of heat around the outside.
The first or inner zone consists of unburned gas given off from the
wick of the candle. The melted grease is drawn up by a capillary
action into the wick and is there converted into gas by the heat of
the flame. With care a portion of this gas can be drawn off through
a tube.
Hold one end of the glass tube in the flame and directly over the
wick. Hold the tube slanting upwards so that the other end is out at
the side and a little above the flame. If held correctly smoke
will come from the end of the tube and can be lighted with a
match.
That it is relatively cool inside of the flame can be shown by
thrusting a match stick into this zone for a few seconds. The
portion of the stick which was held in the dark zone will not be
burned as soon as that portion passing through the sides of the
flame.
EXPERIMENT 302 - The structure of
flame - Manufacturing lampblack
The second or bright yellow zone of the flame contains particles of
carbon heated to a white heat so that they glow brightly. The carbon
is formed by the action of the heat on the gas of the inner
zone.
The presence of this carbon can be shown by holding a cold spoon or
piece of glass tubing in the flame for about a minute. You will
notice that when you take it out it is covered with a black deposit
of lampblack or soot which is one form of carbon. The
cold spoon chills the flame and prevents the carbon from being
completely burned.
Lampblack is made on a large scale in just this way except that
natural gas is burned instead of candles and the cooling is done by
means of iron pipes with water circulating through them.
The third or outside zone of the flame consists of the gases formed
by the complete burning of the carbon particles. lf you will hold
the cold spoon in the outer zone you will find that it gets very hot
but that no soot or only a very small amount will be
deposited. This zone is above the luminous one.
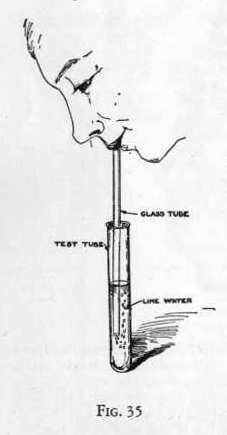
EXPERIMENT 303-Carbon dioxide in
the breath
Make up a solution of lime water as previously directed.
Now take a hollow glass tube, put one end into the test tube
containing the lime water and allow your breath to bubble through
the lime water. (Figure 35) Notice that very soon the water
becomes turbid and after a short while a white precipitate is
formed. This precipitate is calcium carbonate and is formed by
the action of carbon dioxide in the breath upon lime water or
calcium hydroxide.
TANNIN
AND ITS APPLICATIONS.
The raw skin obtained from an animal must be chemically treated to
become useful as leather or fur, otherwise it becomes shriveled and
horny when dry, or, if wet, it is attacked by bacteria which causes
putrefaction and decay.
GILBERT
CHEMISTRY 119
The chemical treatment is called tanning, and the chemical which
combines with the raw skin to change it to leather is called a
tannin.
There are many tannins from different sources which are alike only
in their ability to convert raw skin into leather. Simple treatment
with salts like alum and with oils may suffice for furs. Chromium
compounds are used in chrome tannage, and complex compounds produced
by the action of formaldehyde and sulphuric acid on phenols are
known as synthetic tannins or "syntans" but have their use
limited by cost. The principle tannins are extracted from
trees and plants, sometimes from the bark, sometimes from wood,
leaves, or fruit. The bark of oak and hemlock and the wood of the
chestnut tree have long been used, but one of our most important
sources of tannin is the wood of the quebracho tree which grows in
South America. Your chemical set contains a supply of purified
tannin called tannic acid.
Tannin is unpleasant to taste for it puckers the mouth, as anyone
knows who has tasted a green persimmon. If you follow the directions
given below you can easily test various plant materials to see if
they contain tannin. Possibly you may discover a valuable new
source of tannin in some ordinary weed.
The tanning of skins to make leather is too long a process to
describe here but there are many other applications of tannin which
make interesting experiments.
EXPERIMENT 304 - Testing for tannin
Weigh out one spoonful of ordinary gelatin such as is used in the
kitchen for making desserts. Also weigh out seven spoonfuls of
sodium chloride (common salt) and dissolve them together in 10 test
tubes of water. This is your test solution. It spoils quickly
just as moist raw skins do unless you add a drop or two of a
suitable antiseptic such as carbolic acid.
Now to see what happens when tannin is present, make up a solution
of one measure of tannic acid in one test tube of water. Fill a test
tube about one-third full with the gelatin solution and add the
tannin solution drop by drop. The cloudiness which develops shows
that the tannin has made the gelatin insoluble in water. This is not
only a very sensitive test for tannin, but it shows what happens
when tannin changes raw skin substances to leather.
Raw skin is chemically very similar to gelatin, so when it is soaked
in water containing tannin a similar precipitation takes place
within the structures of the piece of skin, converting it from a
soft jelly-like consistency to a firm tough texture.
EXPERIMENT 305 - Another test for
tannin
Make up a test solution by dissolving one measure of ferric ammonium
sulphate in 10cc. of water. Add a drop of this to a one-quarter
test tube of your tannin solution. The black color which forms
is an indication that tannin is present. Try it on a more dilute
tannin solution. Is the test very sensitive? Some
tannins produce a dark green color with iron instead of black.
You will find elsewhere in this book how you can make use of this
color change to produce ink and perform various mysterious tricks.
EXPERIMENT 306 - Test for tannin in
tea
Drop a pinch of dry tea in a test tube, cover it with water, and
heat nearly to boiling. Now carefully pour off the clear tea
extract from the leaves and add a drop or two to a little of the
gelatin test solution. Also add a drop or two of ferric alum
solution to some of the tea extract in another test tube. Do you
find any tannin in tea?
EXPERIMENT 307 - Tannin from oak
bark
Obtain some oak bark and cut it up into fine shavings. Put
some of these shavings in a test tube half full of water and boil
for two or three minutes. Allow the test tube
120
GILBERT CHEMISTRY
to cool and pour the liquid into another test tube. Test as above
for tannin in the extract.
EXPERIMENTS 308, 309, 310, 311, 312
- Tannin from hemlock bark, chestnut bark, chestnut wood, sumac
leaves, acorns
Follow the directions of the previous experiment (307) with these
materials. Many other common trees and plants contain tannin
in varying amounts. Try extracting tannin from some of your native
trees, shrubs, and weeds. The tannin content varies in different
parts of the same tree. Usually it is present in higher percentage
in the bark, but frequently the leaves, fruits or nuts, and the
heartwood are rich in tannin.
Tannin is important in other ways besides its use in making leather.
Oak timbers owe much of their resistance to rotting to their tannin
content. Many shrubs are not eaten by grazing animals, except as a
last resort to keep from starving, because of the taste of the
tannin in the leaves. Some tannin is used in the textile industry to
assist in dyeing. This use is illustrated by experiments in another
part of your handbook.
Tannin has long been used in medicine, and this application is
increasing greatly since it has been discovered that it is
especially valuable for treating burns.
EXPERIMENT 313 - Making a tannic
acid solution for burns
Dissolve about 2 measures of tannic acid in four test tubes of
water. For small burns, saturate a small pad of cotton or
gauze with this solution and hold it in place over the burn with a
loose bandage. Very large and severe burns are treated by
bathing in the tannin solution or applying the solution as a spray.
In an emergency if your supply of tannic acid is used up, you can
extract enough tannin from tea, as described above in experiment
506. Simply extract the tea leaves with hot water, using plenty of
tea, and use the clear water extract to treat the burn.
PAINTS,
LACQUERS, AND WATER COLORS
In all of these you will find a solid substance furnishing body and
color with a liquid called the vehicle. It is the nature of the
vehicle which makes the chief difference between paints, lacquers,
and water colors.
In paints the vehicle is an oil such as linseed oil which is capable
of combining with oxygen of the air to become hardened to a tough
film. When such an oil has been mixed with a solid such as lead
carbonate or zinc oxide it produces a good weather-resisting paint.
In this case the paint will be white, but suitable coloring matters
called lakes and pigments may be added to give any desired
color.
If these solid colors are ground up with water containing a little
glue or gum to make them stick, water colors are produced. White
wash and calsomine are common examples. These are not usually
weatherproof enough to be used outdoors, but are popular for
inexpensive painting of inside walls.
Lacquers may be considered a kind of varnish, differing from the
older types of varnishes and paints in that the vehicle does not
consist of an oil which dries by oxidation, but is some form of gum
or resin dissolved in a mixture of solvents, which evaporate to
deposit the gum, together with any pigments and coloring matter, in
a tough film. The lacquers may be made very quick~drying by using
solvents which evaporate quickly, but if they dry too quickly the
film is brittle and does not hold well to any surface it is to
cover. To remedy this, a small proportion of a substance called a
plasticizer is added. This is a comparatively non-volatile substance
like castor
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook