 The
Science Notebook
The
Science NotebookGilbert Mineralogy - Part II
Home Terms of Use Safety Contact Us
Experiment
Pages Downloads
Supplies
Useful
Links!

 The
Science Notebook
The
Science Notebook Home Terms of Use Safety Contact Us
Experiment
Pages Downloads
Supplies
Useful
Links!

The more important common minerals are classified into two groups
according as they have large economic importance as ores or are
useful in the arts or are important as rock forming minerals. Let
us now examine a few of these minerals and see if we cannot learn
a little more about them.
Composition - Sulphide
of lead, PbS. Sulphur = 13.4 per cent, lead = 86.6 per cent. Often
carries small amounts of silver sulphide and is sometimes valuable
as a silver ore. When it contains enough silver to be worth
extracting it is called ďargentiferous galena." Whether galena
contains silver or not can be told only by chemical analysis.
Galena also contains at times small traces of zinc, cadmium,
antimony, iron. copper, bismuth, selenium and gold.
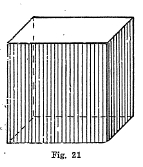
Crystal System - Common form is the cube. (Fig. 21).
Structure - Occurs commonly crystallized or massive cleavable; also occurs coarse or fine grained.
Experiment 1. How to Test for Galena - Powder some of the mineral by breaking it up in a small cloth bag by means of a hammer. Now mix a small amount of the powdered mineral with an equal amount of sodium carbonate and heat the mixture on charcoal in the reducing flame of the blowpipe. Notice that a metallic globule of lead is formed, which is bright lead color when hot but covered with a dull oxide coating when cold. Notice that a coating of lead oxide is also formed, which is yellow near the globule and white farther away. Remove the lead globule and notice that it is malleable and can be hammered out into a thin sheet.
Place a little of the powdered mineral in an open tube and heat slowly over a gas flame if such is handy. Do you recognize the odor of burning sulphur? Nearly all sulphides when heated this way or when roasted on charcoal in the oxidizing flame give this same characteristic pungent odor of sulphur dioxide.
Galena is recognized chiefly by its cubic cleavage, high specific
gravity or weight, softness and black streak.
Occurrence - A common metallic sulphide and found associated with such minerals as pyrite, chalcopyrite, sphalerite, anglesite, cerussite, quartz, dolomite, calcite, barite and fluorite. Also found with silver minerals and sometimes contains silver in sufficient quantity to make it an important silver ore.
It is found in Missouri occurring in the form of beds. disseminated through limestone and also associated with zinc ores. It is found as lead-silver deposits in Idaho, Utah and Colorado. Important deposits are also found in Germany and England.
Uses - It is the most
important lead ore and an important ore of silver. Metallic lead
finds many important uses, some of which are as follows: as sheets
and pipes; in making shot, bullets and weights, in some alloys as
solder (tin and lead) type metal (antimony and lead) and low
fusing alloys (bismuth, tin and lead) commonly called Wood's
metal; large amounts are used in the form of basic lead carbonate,
commonly known as white lead, in paint making; in the form
of litharge (PbO) and red lead (Pb3O4)
for making fine grades of glass, for glazing earthenware and as
pigments; in the form of lead chromates for making red and yellow
paints; in many industries in the form of lead acetate (sugar of
lead). Galena is also used as a detector in wireless sets.
Composition - Antimony
trisulphide, Sb2S3 Antimony = 71.4 per cent,
sulphur = 28.6 per cent. Occasionally contains silver and gold.
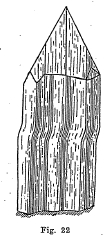
Crystal System - Usually in the form of elongated prisms vertically striated. Crystals sometimes bent or curved and steeply terminated. (Fig. 22.)
Structure - In radiating
crystal groups or in bladed forms with prominent cleavage.
Sometimes massive, coarse to fine granular.
Experiment 2. How to Test for Stibnite - Heat a small piece of the mineral in a gas or candle flame and notice that it fuses or melts very easily.
Heat a small amount of the mineral on charcoal in the oxidizing flame of the blowpipe and notice that a heavy white coating of antimony oxide is formed a short distance from the mineral and the burning mineral gives the irritating odor of burning sulphur.
Heat a little of the powdered mineral in an open tube and notice that two sublimates are formed, one a white non-volatile sublimate on the bottom of the tube and the other a white volatile sublimate in the form of a ring around the tube. Heat a little of the powdered mineral in a closed tube and notice that the mineral gives a faint ring of sulphur and a red deposit of antimony oxysulphide when cold.
Stibnite is usually recognized by its bladed structure, perfect cleavage, soft black streak and lead-gray color.
Occurrence - Found usually in beds or veins of quartz, granite and gneiss. It occurs associated with other antimony ores and with calcite, barite, galena, cinnabar, sphalerite and occasionally gold. Found in China, Japan, Mexico, New South Wales, Saxony, Bohemia and in small deposits in California, Nevada and Idaho.
Uses - Metallic antimony is used in several alloys such as type metal (antimony, bismuth and lead), britannia metal (antimony and tin), babbitt or anti friction metal (antimony, tin and lead) and pewter (antimony and lead). Antimony sulphide is used in fireworks, in safety matches, percussion caps and in the vulcanization of rubber. Antimony trioxide is used as a pigment and in the glazing of enameled ware. Other compounds of antimony like tartar emetic are used in medicine.
Composition - Sulphide of copper and iron, CuFeS2. Sulphur = 35 per cent, copper = 34.5 per cent, iron = 30.5 per cent.
Structure - Occasionally in crystals, but more often massive and compact.
it is attracted by the magnet. Also notice that the globule has the characteristic red copper color and is malleable, for it can be hammered out into a thin sheet.
Heat a small amount of powdered mineral in an open tube and notice the odor of burning sulphur. Heat a little of the powdered mineral in a closed tube and notice the separation of sulphur.
Chalcopyrite is usually recognized by its brass-yellow color. softness and greenish-black streak. It is distinguished from iron pyrites in that it crumbles when cut with a knife while iron pyrites is [sic] not affected by the knife blade. Copper pyrites does [sic] not give off any sparks when struck with steel while iron pyrites does [sic]. It is distinguished from gold in that it is brittle and non-malleable, gold being malleable and soft and easily cut by a knife.
Occurrence - Most common ore of copper. Found widely distributed in metallic veins and associated with such minerals as pyrite, chalcocite, malachite, azurite, sphalerite, galena, quartz, calcite, etc. Sometimes carries gold and silver, in which case it is mined for these metals.
Found widely associated with other copper minerals in the United States, as in Montana, Utah, Colorado, California and Arizona. Also found in England, Spain, Sweden, Canada. Mexico, Alaska, Chili, etc.
Uses - Most important
copper ore. Copper has many varied and important uses as follows:
in wire, sheet and nails; in various alloys as brass (copper and
zinc), German silver (copper, zinc and nickel), bronze (copper,
tin and zinc), gold coin, silver coin, and nickel coin. Copper
sulphate, commonly known as blue vitriol, is used in calico
printing and in galvanic cells.
Composition - Iron disulphide, FeS2 Iron 46.6 per cent, sulphur 53.4 per cent. Contains occasionally small amounts of copper, nickel and cobalt. Sometimes carries small amounts of gold and is then called auriferous pyrite.
Experiment 4. How to Test for Pyrite - Heat a small fragment of the mineral, held in the forceps in the reducing flame of the blowpipe and notice that the mineral fuses When the fragment has become cold, test it with the magnet. Notice that the fragment is magnetic and is attracted by the magnet.
Break a little of the mineral up into the form of a powder and heat a little of the powder in a closed tube, Notice the formation of a large amount of sulphur in the tube. Heat a little of the powder in an open tube. Do you recognize the odor of burning sulphur? This same odor is obtained when a little of the powdered mineral is heated on charcoal in the oxidizing flame.
Make a loop on one end of nickel-steel wire by bending it around the sharpened end of a lead pencil. Heat the loop in the oxidizing flame of the blowpipe for several moments. Then dip the loop into borax and again heat carefully in the blowpipe or gas flame. Notice that the borax puffs up and soon melts. On cooling, you should have a thin, clear transparent bead. Now place a bit of the powdered mineral about the size of a pin head on the bead and heat the bead for several minutes in the reducing flame. Notice that the bead on cooling is bottle green in color. If heated in the oxidizing flame. the bead would be yellow on cooling. This is the borax bead test for iron.
Pyrite is usually distinguished from gold in that it is brittle; from chalcopyrite by the fact that it cannot be scratched by a knife and is of paler color.
Occurrence - Pyrite is a very common occurring mineral and is found in many parts oi the world. It occurs commonly as a vein mineral and is found widely as an accessory mineral in several kinds of rocks. It occurs associated with many different minerals. Important deposits of pyrite are found in Virginia, New York, Massachusetts, California, Portugal and Spain.
Uses - Pyrite is frequently mined because of the gold or copper that is found associated with it. It is never mined as an iron ore because of the large amount of sulphur which it contains. It has an important use, however,in the manufacture of sulphuric acid and sulphate of iron or ferrous sulphate (copperas). Sulphuric acid is probably the most important technical acid and finds many uses in the industries, such as manufacture of other mineral acids, preparation of fertilizers and as a dehydrating or drying agent in many chemical reactions. Sulphur dioxide obtained by burning or roasting pyrite finds an important use as a bleaching agent in the preparation of wood pulp in paper making. Ferrous sulphate is used in the manufacture of inks, in dyeing, in preserving wood and as a disinfectant. Pyrite is also used as a detector in wireless sets.
Commonly known as magnetic iron ore. The name was probably
derived from a locality in Macedonia called Magnesia or to a
shepherd named Magnes who is said to have discovered the mineral
by noticing that the nails of his shoe and the ferrule of his
staff adhered to the ground over which he walked.
Composition - Oxide of iron Fe3O4. Iron = 72.4 per cent, oxygen = 27.6 per cent. The ferrous iron is sometimes replaced by small amounts of magnesium or titanium.
Experiment 5. How to Test for Magnetite - Magnetite cannot be fused or melted in the blowpipe flame. Try fusing a small fragment in the reducing flame of the blowpipe.
Break a little of the mineral up into the form of a powder and. make a borax bead test as described under the mineral pyrite. Notice that in the oxidizing flame the bead is yellow, while in the reducing flame the bead is bottle-green.
Try the effect of the magnet on one or two fragments of the mineral and notice that the mineral is readily attracted by the magnet.
Magnetite is recognized chiefly by its strong magnetism. its hardness, black color and black streak.
Occurrence - Common ore of iron. Found in many rocks and in some cases in the form of large ore bodies. Also occurs in beds and sometimes found in the black sands of the seashore. Often associated with such minerals as corudum, mica, diabase, gabbro and peridotite.
Found in large beds in Northern New York State. Also found in Pennsylvania. New Jersey and England. It constitutes the chief iron ore in Norway and Sweden. The variety known as lodestone is found in Siberia, Germany and Arkansas.
Use - 1mportant iron ore.
Named from the Greek word meaning fire and wash, because of its power of removing the colors from green or brown glass.
Composition - Manganese dioxide, MnO2. Sometimes contains a little water.
Experiment 6. How to Test for Pyrolusite - Heat a small piece of the mineral, held in the forceps, in the blowpipe flame and notice that the mineral is infusible and does not melt.
Make a borax bead as described under the tests for the mineral pyrite and heat a small amount of the mineral about the size of a pinhead on the bead in the oxidizing flame of the blow-pipe. Notice after cooling that the bead is colored reddish-violet. If heated this way in the reducing flame, the bead would be colorless.
Repeat the above test, using a sodium carbonate bead in place of the borax bead. The sodium carbonate bead is made similar to the borax bead. Notice that the mineral imparts a bluish green opaque color to this bead when heated in the oxidizing flame.
Place a small amount of the powdered mineral in a closed tube and heat over a hot flame. While heating, insert into the tube a toothpick or match which has a spark on one end. Notice that the spark glows brightly and in some cases takes fire and burns with a flame. This is because the mineral gives off oxygen when heated.
Occurrence - Found in beds or nests as manganese ores enclosed in clays. Also found occurring in veins with quartz and various metallic minerals.
Occurs in Virginia, California, Arkansas, Georgia, Australia, Japan, India, Nova Scotia, etc.
Uses - Most important ore of manganese. Manganese is used chiefly in the form of alloys, the most important being those containing iron and manganese. The alloy known as spiegeleisen contains iron and manganese and is used extensively in the manufacture of steel. Manganese dioxide is used as an oxidizing agent in the manufacture of chlorine, bromine and oxygen; as a decolorizer of glass; as a drier in paints, and in the dry-cell battery. Potassium permanganate is used as a disinfectant. Manganese is also used for coloring glass, pottery and bricks and in calico printing.
Commonly known as zinc blende or black jack. The name blende is German, meaning blind or deluded because it resembles galena. Sphalerite is Greek, meaning treacherous.
GILBERT MINERALOGYComposition - Zinc sulphide, ZnS. Zinc = 67 per cent, sulphur = 33 per cent. Part of the zinc is sometimes replaced by iron and often small amounts of cadmium, manganese, etc.
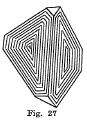
Crystal System -Tetrahedrons are the common form. (Fig. 27). Also found in the form of cubes. Crystals are often twinned and modified so that is is difficult to determine the forms present.
Structure - Usually massive and compact, coarse to fine granular but sometimes botryoidal or fibrous.
Experiment 7. How to Test for Sphalerite - Heat a small fragment of the mineral in the reducing flame of the blowpipe and notice that the mineral is infusible or nearly so.
Break a little of the mineral up into the form of a powder and heat a small amount of the powder on charcoal in the oxidizing flame of the blowpipe. Then heat in the reducing flame. Notice the odor of burning sulphur and the formation of a coating of zinc oxide which is yellow when hot and white when cold. If this coating is moistened with a drop oi cobalt nitrate solution and heated in the reducing flame, it will turn dark green in color.
Mix a little of the powdered mineral with a little sodium carbonate and charcoal and heat on charcoal in the reducing flame of the blowpipe. Notice the bluish green flame that is produced by the mineral. This is the flame color test for zinc.
Sphalerite is usually recognized by its characteristic resinous luster and perfect cleavage.
Uses - Most important zinc ore. Metallic zinc is used chiefly in making brass (an alloy of copper and zinc), in making galvanized iron, in electric batteries and as sheet zinc. The compound zinc oxide or zinc white is used extensively in making paints. Zinc sulphate is used in medicine and in dyeing.
Zinc chloride is used as a preservative for wood. The metal cadmium is often obtained from sphalerite.
Commonly called green copper carbonate. Name is derived from the Greek word mallows on account of its green color.
Composition - Basic
carbonate of copper (CuOH)2CO3. Copper =
57,4 per cent, water = 8.2 per cent, cupric oxide = 71.9 per cent,
carbon dioxide = 19.9 per cent.
Crystal System - Crystals usually found in slender prismatic forms.
Structure - Commonly radiating fibrous with botryroidal or stalactitic surface (Fig. 28). Also found granular or earthy.
Experiment 8. - How to Test for Malachite - Heat a small fragment of the mineral, held in the forceps, in the blowpipe flame and notice that it fuses or melts, giving an emerald green flame. ∑
Break a small amount of the mineral up into a powder and place a little of the powder in a closed tube. Heat the tube over a hot flame and notice that water is given off which condenses in the form of drops on the sides of the tube. Also notice that the mineral turns black.
Mix a little of the powdered mineral with a little sodium carbonate and borax and heat the mixture on charcoal in the reducing flame of the blowpipe. Notice the formation of red metallic globules of copper.
Prepare a borax bead as described under the mineral pyrite and heat a little of the powdered mineral about the size of a pinhead on the bead in the blowpipe flame for several minutes. Notice that on cooling the bead is colored green. This is the borax bead test for copper.
Malachite is recognized generally by its green color and radiating fibrous structure.
0ccurrence - Usually occurs in copper veins that are found in limestones. An important and widely distributed ore of copper. Found in the Ural Mountains of Russia. Africa, Chile. Arizona and New Mexico.
Uses - Important copper ore. Used somewhat as ornamental material for vases and veneer for table tops.
Commonly called calc spar or carbonate of lime. Name derived from Latin word calx, meaning lime.
Composition - Calcium carbonate, CaCO3. Calcium oxide = 56.0 per cent. Carbon dioxide = 44.00 per cent. Calcium sometimes replaced by small amounts magnesium, iron, manganese and zinc.
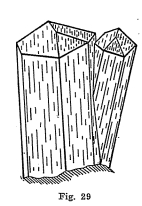
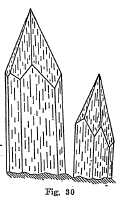
Crystal Systems - Good crystals very common. Figures 29 and 30 illustrate two of the more important types of crystallization. Some crystals are very complex.
Structure - Found fibrous, stalactitic, coarse to fine granular, compact and earthy.
Experiment 10. How to Test for Calcite - Heat a fragment of the mineral, held in the forceps, in the reducing flame of the blowpipe for several minutes and notice that it does not fuse or melt. Moisten a piece of yellow turmeric paper and touch it to the fragment that has been heated. Notice that the turmeric paper turns brown. This is a test for calcium oxide, which gives an alkaline reaction and is present in calcite. If this fragment is moistened with hydrochloric acid and heated, it gives an orange-red flame which is the flame color test for the metal calcium. Small pieces of the mineral also effervesce or give off the gas carbon dioxide, freely, when dissolved in cold hydrochloric acid.
Occurrence - Calcite is a very common and widely diffused mineral. It occurs in very large masses in many rocks. The common rock materials known as limestones, marbles, chalk, calcerous marls, etc., consist essentially of calcium carbonate. A large amount of these carbonates has been formed by the gradual deposition of shells and the skeletons of sea animals which consist mainly of calcium carbonate. It is found also as a vein mineral occurring with all sorts of metallic ores.
Calcite in its various forms is found widely distributed in many localities. Some notable deposits occur in England, Iceland, Mexico, Missouri, Michigan, and New York.
Uses - Calcite is used
chiefly in the manufacture of lime for mortar and cement. By
heating limestone to about 1000 degrees Fahrenheit, carbon
dioxide, a gas, is driven off and the limestone is converted into
quicklime (CaO). White-
wash is made by mixing quicklime with water, while mortar is made by mixing it with sand and water. Portland cement is made by mixing lime with silica and alumina.
Chalk is used for whiting, whitewash, crayons and as a fertilizer. Large deposits of it are found in the famous chalk cliffs of Dover, England.
Limestone is used chiefly as a building material. Also as a flux for smelting certain metallic ores. The chief occurrences in the United States are in Indiana, Pennsylvania, Illinois, Ohio, New York, Missouri and Wisconsin.
Marbles are used primarily as ornamental and building material. Important quarries of marble are found in Vermont, New York, Georgia and Tennessee.
Iceland spar is a valuable variety and is used for making optical instruments in the form of the Nicol prism to produce polarized light. Found in Iceland.
Composition - Calcium fluoride CaF2. Fluorine = 48.9 per cent, calcium = 51.1 per cent.
Experiment 11. How to Test for Fluorite - Heat a small fragment of the mineral, held in the forceps, in the blowpipe flame and notice that it fuses or melts. Also notice that the mineral colors the flame orange. This is the flame color test for the metal calcium. Moisten a small piece of turmeric paper with water and place it on the fragment that has been heated. Notice that the yellow turmeric paper turns brown. This is a test for an alkali, the alkali present being calcium oxide (CaO).
Test for fluorine is as follows: break a little of the mineral up
into the form of a powder and mix a small amount of the powdered
mineral with an equal amount of sodium bisulphate. Place some of
this powdered mixture, about 1/2 inch, in a closed tube and heat
over a hot flame, preferably a gas flame. Notice that after a
short while a white sublimate or deposit of silicon dioxide is
formed on the walls of the tube. When the mixture was heated,
sodium bisulphate was converted into sodium sulphate and sulphuric
acid. The sulphuric acid reacted with the mineral calcium fluoride
to form hydro-
fluoric acid which in turn acts upon the glass and etches it. A secondary reaction then sets in with the formation of a white deposit of silica on the glass tube. This is the test that is usually applied to the element fluorine,
Fluorite is usually recognized by its cubic crystals, octehedral cleavage, vitreous luster, color and by its being scratched with a knife.
Occurrence - Common mineral and widely distributed. Usually found in veins or associated with metallic ores such as tin and lead. Also associated with dolomite, limestone, calcite, gypsum, barite, galena, quartz, cassiterite, sphalerite, topaz, apatite, etc.
Fluorite is found as important deposits in Illinois and Kentucky. Also in England and Saxony.
Uses - The better grade of fluorite is used in the manufacture of opalescent glass. Other grades are used in enameling cooking utensils, as a flux in steel making, in the preparation of hydrofluoric acid and sometimes as ornamental material in the form of vases and dishes.
Composition - Sodium chloride, NaCl. Sodium = 39.4 per cent, chlorine = 60.6 per cent. Often contains impurities such as calcium and magnesium chlorides and calcium sulphate.
Break a little of the mineral up into the form of a powder and
try to dissolve a little of the powder in some water in a test
tube, Notice that the mineral dissolves readily in water. Taste a
little of this solution. Do you recognize the salty taste? If this
solution was treated with a solution of silver nitrate you would
obtain a white precipitate of silver chloride. This is the test
for chlorine in a soluble compound.
Salt beds were originally formed by the gradual evaporation and drying up of salt water which had been shut out from the sea. These beds have then become covered by other deposits of sediment so that salt beds are found to a depth of two thousand feet below the surface of the ground. These salt beds range from a few feet to a hundred feet in thickness.
Salt is produced in the United States either by mining by means of shafts and galleries or by pumping the salt brine from the salt bed to the surface and evaporating off the water, Many important deposits are found in the United States, especially in New York, Michigan, Ohio, Kansas and Louisiana. Important deposits are found also in Great Britain, Poland, Hungary, Germany and Spain.
Uses - Rock salt is used
chiefly for culinary and preservative purposes. Also used in
manufacture of sodium carbonate for glass making, soap making and
bleaching. Also used in the preparation of sodium compounds and in
the extraction of gold by the chlorination process.
The rock making minerals are in most cases complex silicates of such metals as aluminum, magnesium, calcium, iron, sodium, potassium and hydroxyl (OH). The physical properties of these minerals usually afford sufficient means for their identification.
Commonly known as potash feldspar. The name orthoclase refers to the right-angled cleavage which the mineral has. Feldspar is taken from the German word feld, meaning field. Orthoclase belongs to a class of rock-making minerals called the feldspars. They are complex silicates of aluminum with sodium, potassium or calcium and sometimes barium. .
Composition - Potassium aluminum silicate, KAlSi3O8. Aluminum oxide = 18.4 per cent, potash = 16.9 per cent and silicon dioxide = 64.7 per cent. Soda sometimes replaces part of the potash.
Experiment 13. How to Test for Orthoclase - Heat a small splinter or fragment of the mineral in the reducing flame of the blowpipe and notice that the mineral does not fuse or melt easily. It melts only on the edges of thin fragments.
If the powdered mineral is mixed with powdered gypsum and heated on platinum or nickel-steel wire, the flame will be colored purple, which is the flame test for the mineral potassium.
Orthoclase is usually recognized by its hardness, color and cleavage.
0ccurrence - A very common rock-forming mineral. It occurs widely distributed in all types of rocks and is found associated with the minerals quartz, albite and muscovite. It is largely a vein mineral and is found in New England and Middle Atlantic States, chiefly in Connecticut, Maine, New York, Maryland and Pennsylvania. Feldspar is quarried in large amounts in some of these localities.
Uses - Orthoclase finds an important use in the manufacture of porcelain. The finely ground mineral is mixed with clay (kaolin) and quartz and, when heated to a high temperature, the feldspar fuses and acts as a cement to bind the material together. Fused feldspar is one of the chief ingredients of the glaze on porcelain.
Commonly called steatite or soapstone.
Composition - Si1icate of magnesium, H2Mg3(SiO3)4. Magnesium oxide = 31.7 per cent, silicon dioxide = 63.5 per cent, water = 4.8 per cent.
Structure - Usually massive. with foliated structure. Can be split into thin plates, which are flexible but not elastic when found in this form. Also compact.
Crystal System - Crystals rare.
Experiment 14. How to Test for Talc - Heat a small piece of the mineral, held in the forceps, in the reducing flame of the blowpipe and notice that it is difficult to fuse or melt the mineral. It fuses to an enamel on the edges only.
If a small piece of the mineral is moistened with a solution of cobalt nitrate and heated in the blowpipe flame the mineral will become pale violet in color.
Talc is usually recognized by its resemblance to mica in structure and cleavage, and by its softness and greasy feel.
Occurrence - Talc is
usually found as a mineral formed by the alteration of magnesium
silicates such as chrysolite, pyroxene, amphibole, etc.
Soapstone quarries are found in the United States chiefly in Vermont, Massachusetts, Rhode Island. New York, New Jersey, Pennsylvania, Maryland, North Carolina, Virginia and Georgia.
Uses - Soapstone has many uses. In the form of slabs it is used extensively for making wash tubs, sinks, table tops, electrical switchboards, furnace linings, hearthstones, etc, The compact variety is used as tailorís chalk, slate pencils, gas burners, etc. Finely divided talc is used in paper making as a filler to give weight, as a lubricant, as toilet powders (talcum powder, in paints, as a heat insulator and in several other things.
Name taken from the Greek word apatao, meaning to deceive.
Composition - Phosphate and fluoride of calcium, Ca4(CaF) (PO4)3 or phosphate and chloroide of calcium (Ca4)(CaCl) (PO4)3. The first is called fluor-apatite while the second is called chlor-apatite.

Experiment 15. How to Test for Apatite - Heat a small fragment of the mineral, held in the forceps, in the reducing flame of the blowpipe for several minutes and notice that it does not fuse or melt easily. Notice the orange colored flame produced by the mineral when heated in this way. This is the flame test for the metal calcium.
The powdered mineral dissolves in concentrated hydrochloric acid and gives a white precipitate of calcium sulphate when a drop or two of sulphuric acid is added.
The mineral is usually recognized by its hardness, color and crystal forms.
Uses - Apatite is used chiefly as a fertilizer on account of the phosphorous which it contains, Transparent varieties are used somewhat as gems.
Also known as muscovy-glass or common mica. The name was derived
from the mineral muscovy-glass which was used as a substitute for
glass in Russia. Mica is derived from the Latin word micare,
meaning to shine.
Composition - A complex silicate of potassium, aluminum and hydrogen, H2KAl3(SiO4)3. Frequently contains traces of iron, magnesium, calcium, sodium, lithium, fluorine and titanium.
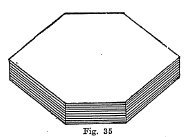
Crystal System - Usua1ly in the form of six-sided plates (Fig. 35), Also massive and in scales.
Structure - Large and small sheets foliated. Sometimes in scales. Distinct crystals rare.
Experiment 16. How to Test for Muscovite - Heat a small piece of the mineral, held in the forceps, in the blowpipe flame for several minutes and notice that the mineral does not fuse or melt very readily.
Muscovite is usually recognized by its light color and mica-like structure.
Occurrences - A very common and widely distributed rock-making mineral. It is found in many types of rocks. Muscovite is found in veins associated with quartz, feldspar, tourmaline, beryl, garnet, fluorite and apatite.
Muscovite is found in the United States chiefly in the Appalachian and Rocky Mountain regions. It occurs in pegmatite veins in North Carolina and South Dakota. Deposits are also found in Connecticut, Maine, New Hampshire, Colorado, Alabama and Virginia. Also found in large deposits in Canada and India.
Uses - Mica is used extensively as an insulating material in the construction of electrical apparatus. Used to take the place of windows (isinglass), for stove doors, lanterns, etc. Powdered mica is used to give wall paper a shiny luster, as a non-conductor of heat, as a lubricant in oils, and as a fireproofing material.
The garnets, of which there are six varieties, are complex silicates of the metals calcium, magnesium, iron, manganese, aluminum and chromium. The name garnet is derived from the Latin word granatus, meaning like a grain.
Composition - The variety of garnet known as almandite is a silicate of iron and aluminum, Fe3Al2(SiO4)3. The other varieties of garnet have a similar a formula with the iron and aluminum replaced by some of the other metals mentioned above.
| Element | Symbol | Atomic Wgt |
Valence |
| Aluminum Antimony Argon Arsenic Barium Bismuth Boron Bromine Cadmium Calcium Carbon Chlorine Chromium Cobalt Copper Fluorine Gold Helium Hydrogen Iodine Iron Lead Lithium Magnesium Manganese Mercury Nickel Nitrogen Oxygen Phosphorous Platinum Potassium Silicon Silver Sodium Strontium Sulphur Tin Zinc |
Al Sb A As Ba Bi B Br Cd Ca C Cl Cr Co Cu F Au He H I Fe Pb Li Mg Mn Hg Ni N O P Pt K Si Ag Na Sr S Sn Zn |
27 120 40 75 137 208 11 80 112 40 12 35 52 59 63 19 197 4 1 127 56 207 7 24 55 200 59 14 15 31 195 39 28 108 23 87 32 119 65 |
3 3, 5 3, 5 2 3, 5 3 1 2 2 4 1 2, 3, 4 2 1, 2 1 1, 3 1 1 2, 3 2, 4 1 2 2, 4 1, 2 2 3, 5 2 3, 5 4 1 4 1 1 2 2, 4, 6 2, 4 2 |
| Albite Amphibole Apatite Arsenopyrite Barite Biotite Bornite Calamine Calcite Cassiterite Celestite Cerussite Chalcocite Chalcopyrite Cinnabar Corundum Cuprite Dolomite Epidote Fluorite Galena Garnet Graphite Halite Hematite Kaolinite Limonite Magnetite Malachite Muscovite Natrolite Orthoclase Pyrite Pyrolusite Pyroxene Quartz Serpentine Siderite Smithsonite Sphalerite Staurolite Stibnite Talc Tetrahedrite Tourmaline |
sodium aluminum silicate calcium and magnesium silicate fluoride and phosphate of calcium sulpharsenide of iron barium sulphate complex silicate of aluminum, iron, magnesium and potassium copper iron sulphide zinc silicate calcium carbonate tin oxide strontium sulphate lead carbonate cuprous sulphide iron and copper sulphide mercuric sulphide aluminum oxide cuprous oxide calcium magnesium carbonate complex silicate of calcium, aluminum and iron. calcium fluoride lead sulphide complex silicate of iron, magnesium, aluminum and calcium carbon sodium chloride iron oxide complex aluminum silicate iron oxide iron oxide basic copper carbonate complex silicate of potassium and aluminum complex silicate of sodium and aluminum potassium aluminum silicate iron disulphide manganese dioxide calcium magnesium silicate silicon dioxide magnesium silicate iron carbonate zinc carbonate zinc sulphide complex silicate of iron and aluminum antimony sulphide magnesium silicate antimony copper sulphide complex silicate of boron and aluminum |
NaAlSi3O8 FeAsS BaSO4 Cu5FeS4. CaCO3 SnO2 SrSO4 PbCO3 Cu2S CuFeS2 HgS Al2O3 Cu2O CaMg(CO3)2 CaF2 PbS C NaCl Fe2O3 2Fe2O3 3H2O Fe3O4 CuCO3Cu(OH)2 FeS2 MnO2 SiO2 FeCO3 ZnCO3 ZnS Sb2S3 Cu8Sb2S7 |