 The
Science Notebook
The
Science NotebookHenley's Book of Formulas, Recipes and Processes
Home Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!
Henley's Twentieth Century Book of Formulas, Recipes and Processes - Pages 676-700
[676]
SPIRIT
of the malt the temperature may be raised to that of boiling water. If, however, the malt be added before the mashing begins, the temperature should not rise much, if any, above 140º F., since the fermenting power is retarded and disturbed at higher temperatures. The mashing is simply a mechanical process by means of which the starch is reduced to a form of paste and the temperature maintained at that point which is best suited to the conversion of the starch into sugar.
Fermentation. The mash, after the starch has all been converted into sugar, goes into fermenting tanks, which in Scotland are called "wash backs," when the yeast is added.. A view of the typical wash back is shown in Fig. 3. They often have a stirring apparatus, as indicated in the figure; whereby the contents can be thoroughly mixed with the yeast and kept in motion. This is not necessary after the fermentation is once well established, but it is advisable, especially in the early stages, to keep the yeast well distributed throughout the mass. In these tanks the fermentations are conducted, the temperature being varied according to the nature of the product to be made. For industrial alcohol the sole purpose should be to secure the largest possible percentage of alcohol without reference to its palatable properties.
An organism belonging to the vegetable family and to which the name "yeast" has been given is the active agent in fermentation. The organism itself does not take a direct part in the process, but it secretes another ferment of an unorganized character known as an "enzym" or a "diastase." This enzym has the property, under proper conditions of food, temperature, and dilution, of acting upon sugar and converting it into alcohol and carbonic acid. Anyone who has ever seen a fermenting vat in full operation and noticed the violent boiling or ebullition of the liquor, can understand how rapidly the gas "carbon dioxide" or "carbonic acid," as it is usually called, may be formed, as it is the escape of this gas which gives the appearance to the tank of being in a violent state of ebullition. The yeast which produces the fermentation belongs to the same general family as the ordinary yeast which is used in the leavening of bread. The leavening of bread under the action of yeast is due to the conversion of the sugar in the dough into alcohol and carbon dioxide or carbonic acid. The gas thus formed becomes entangled in the particles of the gluten, and these expanding cause the whole mass to swell or "rise," as it is commonly expressed. Starch cannot be directly fermented, but must be first converted into sugar, either by the action of a chemical like an acid, or a ferment or enzym, known as diastase, which is one of the abundant constituents of malt, especially of barley malt. In the preparation of a cereal, for instance, for fermentation, it is properly softened and ground, and then usually heated with water to the boiling point or above in order that the starch may be diffused throughout the water. After cooling, it is treated with barley malt, the diastase of which acts vigorously upon the starch, converting it into a form of sugar, namely, maltose, which lends itself readily to the activities of the yeast fermentation. (Fig. 4.)
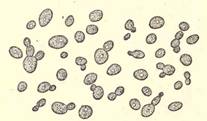
FIG. 4. YEAST FROM BEER SEDIMENT SHOWING BUDDING ( X 1270).
When ordinary sugar (cane sugar, beet sugar, and sucrose) is subjected to fermentation it is necessary that the yeast, which also exerts an activity similar to that of malt, should first convert the cane sugar into invert sugar (equal mixtures of dextrose and levulose) before the alcoholic fermentation is set up. The cane sugar is also easily inverted by heating with an acid.
When different kinds of sugars and starches are fermented for the purpose of making a beverage it is important that the temperature of fermentation be carefully controlled, since the character of the product depends largely upon the temperature at which the fermentation takes place. On the contrary, when industrial alcohol is made, the sole object is to get as large a yield as possible, and for this reason that temperature should be employed which produces the most alcohol and the least by-products, irrespective of the flavor or character of the product made. Also, in the making of alcoholic beverages, it is important that the malt be of the very best quality in
[677]
SPIRIT
order that the resulting product may have the proper flavor. In the production of alcohol for industrial purposes this is of no consequence, and the sole purpose here should be to produce the largest possible yield. For this reason there is no objection to the use of acids for converting the starch, cane sugar, and cellulose into fermentable sugars. Therefore, the heating of the raw materials under pressure with dilute acids in order to procure the largest quantity of sugar is a perfectly legitimate method of procedure in the manufacture of industrial alcohols.
Sugars and starches are usually associated in nature with another variety of carbohydrates known as cellulose, and this cellulose itself, when acted upon by an acid, is converted very largely into sugars, which, on fermentation, yield alcohol. For industrial purposes, the alcohol produced in this manner is just as valuable as that made from sugar and starch. Whether the diastatic method of converting the starch and sugar into fermentable sugars be used, or the acid method, is simply a question of economy and yield. On the other hand, when alcoholic beverages are to be made, those processes must be employed, irrespective of the magnitude of the yield, which give the finest and best flavors to the products.
Distillation. The object of distillation is to separate the alcohol which has been formed from the non-volatile substances with which it is mixed. A typical form of distilling apparatus for the concentration of the dilute alcohol which is formed in the beer or wash from the fermentation tanks, is represented in Fig. 5.
This apparatus is of the continuous type common to Europe and America. It consists of a "beer still" provided with a number of chambers fitted with perforated plates and suitable overflow pipes. It is operated as follows:
The syrup and alcohol are pumped into the top of the beer still through a pipe G; the tank G may also be placed above the center of the still and the contents allowed to flow into the still by gravity; steam is admitted through an open pipe into the kettle A at the bottom of the column or is produced by heating the spent liquor by means of a coil. The steam ascends through the perforations in the plates, becoming richer and richer in alcohol as it passes through each layer of liquor, while the latter gradually descends by means of the overflow pipes to the bottom of the column B and finally reaches the kettle completely exhausted of alcohol, whence it is removed by means of a pump connected with the pipe line H. On reaching the top of the beer still B the vapors of the alcohol and the steam continue to rise and pass into the alcohol column C. This column is also divided into chambers, but by solid instead of perforated plates, as shown at
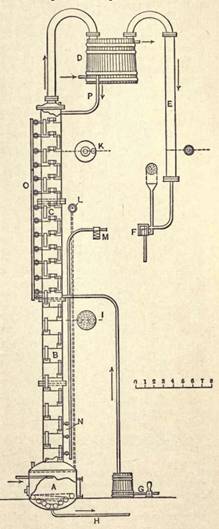
FIG. 5. CONTINUOUS DISTILLING APPARATUS.
K. Each chamber is provided with a return or overflow pipe and an opening through which the vapors ascend. In the alcohol column the vapors are so directed as to pass through a layer of
[678]
SPIRIT - SPONGES
liquid more or less rich in alcohol which is retained by the plate separating the compartments. An excess of liquids in these compartments overflows through the down pipes, gradually works its way into the beer still, and thence to the kettle. On reaching the top of the column the vapors, which have now become quite rich in alcohol, are passed into a coil provided with an outlet at the lowest part of each bend. These outlets lead into the return pipe P, which connects with the top chamber of the alcohol column. This coil is technically termed the "goose" and is immersed in a tank called the "goose tub." A suitable arrangement is provided for controlling the temperature of the water in the tub by means of outlet and inlet water pipes. When the still is in operation the temperature of the "goose" is regulated according to the required density of the alcohol. The object of the "goose" is the return to the column of 'all low products which condense at a temperature below the boiling point of ethyl alcohol of the desired strength. On leaving the "goose" the vapors enter a condenser E, whence the liquid alcohol is conducted into a separatorº F. This separator consists simply of a glass box provided with a cylinder through which a current of alcohol is constantly flowing. An alcohol spindle is inserted in this cylinder and snows the density of the spirit at all times. A pipe, with a funnel-shaped opening at its upper extremity, connects with the pipe leading from the condenser and gives vent to any objectionable fumes. The separator is connected by means of a pipe with the alcohol storage tank. The pipe O is for emptying the upper chambers when necessary. The valves N, communicating by means of a small pipe with a condenser M, are for testing the vapors in the lower chambers for alcohol.
Substances Used for Denaturing Alcohol. The process of rendering alcohol unsuitable for drinking is called "denaturing," and consists, essentially, in adding to the alcohol a substance soluble therein of a bad taste or odor, or both, of an intensity which would render it impossible or impracticable to use the mixture as a drink. Among the denaturing substances which have been proposed are the following:
Gum shellac (with or without the addition of camphor, turpentine, wood spirit, etc.), colophonium, copal rosin, Manila gum, camphor, turpentine, acetic acid, acetic ether, ethylic ether, methyl alcohol (wood alcohol), pyridine, acetone, methyl acetate, methyl violet, methylene blue, aniline blue, eosin, fluorescein, naphthalene, castor oil, benzine, carbolic acid, caustic soda, musk, animal oils, etc.
Methyl (wood) alcohol and benzine are the denaturing agents authorized in the United States, in the following proportions: To 100 parts, by volume, of ethyl alcohol (not less than 90 per cent strength) add 10 parts of approved methyl (wood) alcohol and 1/2 of 1 part of approved benzine. Such alcohol is classed as completely denatured. Formulas for special denaturation may be submitted for approval by manufacturers to the Commissioner of Internal Revenue, who will determine whether they may be used or not, and only one special denaturant will be authorized for the same class of industries unless it shall be shown that there is good reason for additional special denaturants. Not less than 300 wine gallons can be withdrawn from a bonded warehouse at one time for denaturing purposes.
Spirit. Proof spirit is a term used by the revenue department in assessing the tax on alcoholic liquors. It means a liquid in which there is 50 per cent (by volume) of absolute alcohol. As it is the actual alcohol in the whisky, brandy, dilute alcohol, etc., which is taxed, and as this varies so widely, it is necessary that the actual wine gallons be converted into proof gallons before the tax rate can be fixed. A sample that is half alcohol and half water (let us say for convenience) is "100 proof." A sample that is 3/4 alcohol and 1/4 water is 150 proof, and the tax on every gallon of it is 1 1/2 times the regular government rate per proof gallon. Absolute alcohol is 200 proof and has to pay a double tax.
The legal definition of proof spirit is, "that alcoholic liquor which contains one-half its volume of alcohol of a specific gravity of 0.7939 at 60º F."
SPONGES:
Bleaching Sponges.
I. Soak in dilute hydrochloric acid to remove the lime, then wash in water, and place for 10 minutes in a 2 per cent solution of potassium permanganate. The brown color on removal from this solution is due to the deposition of manganous oxide, and this may be removed by steeping for a few minutes in very dilute sulphuric acid. As soon as the sponges appear white, they are washed out in water to remove the acid.
II. A sponge that has been used in
[679]
SPONGES- STAMPING
surgical operations or for other purposes, should first be washed in warm water, to every quart of which 20 drops of liquor of soda have been added; afterwards washed in pure water, wrung or pressed out and put into a jar of bromine water, where it is left until bleached. Bleaching is accelerated by exposing the vessel containing the bromine water to the direct rays of the sun. When the sponge is bleached it is removed from the bromine water, and put for a few minutes in the water containing soda lye. Finally it is rinsed in running water until the odor of bromine disappears. It should be dried as rapidly as possible by hanging it in the direct sunlight.
Sterilization of Sponges.
I. Allow the sponges to lie for 24 hours in an 8 per cent hydrochloric acid solution, to eliminate lime and coarse impurities; wash in clean water, and place the sponges in a solution of caustic potash, 10 parts; tannin, 10 parts; and water, 1,000 parts. After they have been saturated for 5 to 20 minutes with this liquid, they are washed out in sterilized water or a solution of carbolic acid or corrosive sublimate, until they have entirely lost the brown coloring acquired by the treatment with tannin. The sponges thus sterilized are kept in a 2 per cent or 15 per cent carbolic solution.
Sponge Window Display. Soak a large piece of coarse sponge in water, squeeze half dry, then sprinkle in the openings red clover seed, millet, barley, lawn grass, oats, rice, etc. Hang this in the window, where the sun shines a portion of the day, and sprinkle lightly with water daily. It will soon form a mass of living green vegetation very refreshing to the eyes. While the windows are kept warm this may be done at any season. The seeds used may be varied, according to fancy.
SPONGES AS FILTERS:
See Filters.
SPONGE CLEANERS:
See Cleaning Preparations and Methods, under Miscellaneous Methods.
SPONGE TRICK, BURNING:
See Pyrotechnics.
SPOT ERADICATORS:
See Cleaning Preparations and Methods and Soaps.
SPOT GILDING:
See Plating.
SPRAY SOLUTION:
See Balsams.
SPEARMINT CORDIAL:
See Wines and Liquors.
SPRAIN WASHES:
See Veterinary Formulas.
SPRING CLEANING:
See Cleaning Preparations and Methods.
SPRING HARDENING:
See Steel.
SPRINGS OF WATCHES:
See Watchmakers' Formulas.
SPRUCE BEER:
See Beverages.
STAIN REMOVERS:
See Cleaning Preparations and Methods.
STAINS:
See Paints, Varnishes and Wood Stains.
STAINS FOR LACQUERS:
See Lacquers.
Stamping
(See also Dyes.)
Stamping Colors for Use with Rubber Stamps. Blue: 0.3 parts of water-blue 1 B, 1.5 parts of dextrin, 1.5 parts of distilled water. Dissolve the aniline dye and the dextrin in the distilled water, over a water bath, and add 7 parts of refined glycerine, 28º Bé.
Other colors may be made according to the same formula, substituting the following quantities of dyes for the water-blue: Methyl violet 3 B, 0.02 parts; diamond fuchsine I, 0.02 parts; aniline green D, 0.04 parts; vesuvine B, 0.05 parts; phenol black, 0.03 parts. Oleaginous colors are mostly used for metallic stamps, but glycerine colors can be used in case of necessity.
Oleaginous Stamping Colors. Mix 0.8 parts of indigo, ground fine with 2.5 parts of linseed-oil varnish, and 0.5 parts of olein. Add 2 parts of castor oil and 5 parts of linseed oil. For other colors according to the same formula, use the following quantities: Cinnabar, 2 1/2 parts; verdigris, 2 1/2 parts; lampblack, 1.2 parts; oil-soluble aniline blue A, 0.35 parts; oil-soluble aniline scarlet B, 0.3 parts; aniline yellow (oil-soluble), 0.45 parts; oil-soluble aniline black L, 0.6 parts.
Stamping Liquids and Powders. Dissolve 1 drachm each of rosin and copal
[680]
STAMPING - STARCH
in 4 fluidounces of benzine and with a little of this liquid triturate 1/2 drachm of Prussian blue and finally mix thoroughly with the remainder.
Ultramarine, to which has been added a small proportion of powdered rosin, is generally used for stamping embroidery patterns on white goods. The powder is dusted through the perforated pattern, which is then covered with a paper and a hot iron passed over it to melt the rosin and cause the powder to adhere to the cloth. The following are said to be excellent powders:
I. White. One part each of rosin, copal, damar, mastic, sandarac, borax, and bronze powder, and 2 parts white lead.
II. Black. Equal parts of rosin, damar, copal, sandarac, Prussian blue, ivory black, and bronze powder.
III. Blue. Equal parts of rosin, damar, copal, sandarac, Prussian blue, ultramarine, and bronze powder.
In all these powders the gums are first to be thoroughly triturated and mixed by passing through a sieve, and the other ingredients carefully added. Other colors may be made by using chrome yellow, burnt or raw sienna, raw or burnt umber, Vandyke brown, etc. For stamping fabrics liable to be injured by heat, the stamping is done by moistening a suitable powder with alcohol and using it like a stencil ink.
Stamping Powder for Embroideries. "Stamping powders" used for outlining embroidery patterns are made by mixing a little finely powdered rosin with a suitable pigment. After dusting the powder through the perforated pattern it is fixed on the fabric by laying over it a piece of paper and then passing a hot iron carefully over the paper. By this means the rosin is melted and the mixture adheres. When white goods are to be "stamped," ultramarine is commonly used as the pigment; for dark goods, zinc white may be substituted. Especial care should be taken tc avoid lead compounds and other poisonous pigments, as they may do mischief by dusting off. On velvets or other materials likely to be injured by heat, stamping is said to be done by moistening a suitable powder with alcohol and using it as stencil paint. A small addition of rosinous matter would seem required here also.
Starch
Black Starch. Add to the starch a certain amount of logwood extract be fore the starch mixture is boiled. The quantity varies according to the depth of the black and the amount of starch. A small quantity of potassium bichromate dissolved in hot water is used to bring out the proper shade of black. In place of bichromate, black iron liquor may be used. This comes ready prepared.
Starch Gloss.
I. Melt 2 1/2 pounds of the best paraffine wax over a slow fire. When liquefied remove from the fire to stir in 100 drops of oil of citronella. Place several new pie tins on a level table, coat them slightly with sweet oil, and pour about 6 tablespoonfuls of the melted paraffine wax into each tin. The pan may be floated in water sufficiently to permit the mixture to be cut or stamped out with a tin cutter into small cakes about the size of a peppermint lozenge. Two of these cakes added to each pint of starch will cause the smoothing iron to impart the finest possible finish to, muslin or linen, besides perfuming the clothes.
II.
Gum arabic, powdered 3 parts
Spermaceti wax 6 parts
Borax, powdered 4 parts
White cornstarch 8 parts
All these are to be intimately mixed in the powder form by sifting through a sieve several times. As the wax is in a solid form and does not readily become reduced to powder by pounding in a mortar, the best method of reducing it to such a condition is to put the wax into a bottle with some sulphuric or rectified ether and then allow the fluid to evaporate. After it has dissolved the wax, as the evaporation proceeds, the wax will be deposited again in the solid form, but in fine thin flakes, which will easily break down to a powder form when rubbed up with the other ingredients in a cold mortar. Pack in paper or in cardboard boxes. To use, 4 teaspoonfuls per pound of dry starch are to be added to all dry starch, and then the starch made in the usual way as boiled starch.
Refining of Potato Starch. A suitable quantity of chloride of lime, fluctuating according to its quality between 1/2 to 1 part per 100 parts of starch, is made with little water into a thick paste. To this paste add gradually with constant stirring 10 to 15 times the quantity of water, and filter.
The filtrate is now added to the starch stirred up with water; 1/2 part of ordinary
[681]
STARCH - STEEL
hydrochloric acid of 20º Bé. previously diluted with four times the quantity of water is mixed in, for every part of chloride of lime, the whole is stirred thoroughly, and the starch allowed to stand.
When the starch has settled, the supernatant water is let off and the starch is washed with fresh water until all odor of chlorine has entirely disappeared. The starch now obtained is the resulting final product.
If the starch thus treated is to be worked up into dextrin, it is treated in the usual manner with hydrochloric acid or nitric acid and will then furnish a dextrin perfectly free from taste and smell.
In case the starch is to be turned into "soluble" starch proceed as usual, in a similar manner as in the production of dextrin, with the single difference that the starch treated with hydrochloric or nitric acid remains exposed to a temperature of 212º F., only until a test with tincture of iodine gives a bluish-violet reaction. The soluble starch thus produced, which is clearly soluble in boiling water, is odorless and tasteless.
Starch Powder. Finely powdered starch is a very desirable absorbent, according to Snively, who says that for toilet preparations it is usually scented by a little otto or sachet powder. Frangipanin powder, used in the proportion of 1 part to 30 of the starch, he adds, gives a satisfactory odor.
STARCHES:
See Laundry Preparations.
STARCH IN JELLY, TESTS FOR:
See Foods.
STARCH PASTE:
See Adhesives.
STATUE CLEANING:
See Cleaning Preparations and Methods.
STATUETTES, CLEANING OF:
See Plaster.
STATUETTES OF LIPOWITZ METAL:
See Alloys.
Steel
(See also Iron and Metals.)
ANNEALING STEEL:
See also Hardening Steel and Tempering Steel.
This work requires the use of substances which yield their carbon readily and quickly to the tools on contact at a high temperature. Experience has shown that the best results are obtained by the use of yellow blood-lye salt (yellow prussiate of potash), which, when brought in contact with the tool at a cherry-red heat, becomes fluid, and in this condition has a strong cementing effect. The annealing process is as follows: The tool is heated to a cherry red and the blood-lye salt sprinkled over the surface which is to be annealed. A fine sieve should be used, to secure an even distribution of the substance. The tool is then put back into the fire, heated to the proper temperature for tempering, and tempered. If it is desired to give a higher or more thorough tempering to iron or soft steel, the annealing process is repeated 2 or 3 times. The surface of the tool must, of course, be entirely free from scale. Small tools to which it is desired to impart a considerable degree of hardness by annealing with blood-lye salt are tempered as follows: Blood-lye salt is melted in an iron vessel over a moderate fire, and the tool, heated to a brown-red heat, placed in the melted salt, where it is allowed to remain for about 15 minutes. It is then heated to the hardening temperature and hardened. A similar but milder effect is produced in small, thin tools by making them repeatedly red hot, immersing them slowly in oil or grease, reheating them, and finally tempering them in water. To increase the effect, soot or powdered charcoal is added to the oil or grease (train oil) till a thick paste is formed, into which the red-hot tool is plunged. By this means the tool is covered with a thick, not very combustible, coating, which produces a powerful cementation at the next heating. By mixing flour, yellow blood-lye salt, saltpeter, horn shavings, or ground hoofs, grease, and wax, a paste is formed which serves the same purpose. A choice may be made of any of the preparations sold as a "hardening paste"; they are all more or less of the same composition. This is a sample: Melt 500 grains of wax, 500 grains tallow, 100 grains rosin, add a mixture of leather-coal, horn shavings, and ground hoofs in equal parts till a paste is formed, then add 10 grains saltpeter and 50 to 100 grains powdered yellow blood-lye salt, and stir well. The tools are put into this paste while red hot, allowed to cool in it, then reheated and tempered.
More steel is injured, and sometimes spoiled, by over-annealing than in any other way. Steel heated too hot in annealing will shrink badly when being hardened; besides, it takes the life out of it. It should never be heated above a
[682]
STEEL
low cherry red, and it should be a lower heat than it is when being hardened. It should be heated slowly and given a uniform heat all over and through the piece.
This is difficult to do in long bars and in an ordinary furnace. The best way to heat a piece of steel, either for annealing or hardening, is in red-hot, pure lead. By this method it is done uniformly, and one can see the color all the time. Some heating for annealing is done in this way: Simply cover up the piece in sawdust, and let it cool there, and good results will be obtained.
Good screw threads cannot be cut in steel that is too soft. Soft annealing produces a much greater shrinkage and spoils the lead of the thread.
This mixture protects the appearance of polished or matted steel objects on heating to redness: Mix 1 part of white soap, 6 parts of chemically pure boracic acid, and 4 parts of phosphate of soda, after pulverizing, and make with water into a paste. For use, apply this to the article before the annealing.
COLORING STEEL:
Black.
I. Oil or wax may be employed on hard steel tools; with both methods the tool loses more or less of its hardness and the blacking process therefore is suited only for tools which are used for working wood or at least need not be very hard, at any rate not for tools which are employed for working steel or cast iron. The handsomest glossy black color is obtained by first polishing the tool neatly again after it has been hardened in water, next causing it to assume on a grate or a hot plate the necessary tempering color, yellow, violet blue, etc., then dipping it in molten, not too hot, yellow wax and burning off the adhering wax, after withdrawal, at a fire, without, however, further heating the tool. Finally dip the tool again into the wax and repeat the burning off at the flame until the shade is a nice lustrous black, whereupon the tool may be cooled off in water. The wax is supposed to impart greater toughness to the tool. It is advisable for all tools to have a trough of fat ready, which has been heated to the necessary tempering degree, and the tools after hardening in water are suspended in the fat until they have acquired the temperature of the fat bath. When the parts are taken out and slowly allowed to cool, they will be a nice, but not lustrous, black.
II. The following has been suggested for either steel or iron:
Bismuth chloride 1 part
Mercury bichloride 2 parts
Copper chloride 1 part
Hydrochloric acid 6 parts
Alcohol 5 parts
Water sufficient to make 64 parts.
Mix. As in all such processes a great deal depends upon having the article to be treated absolutely clean and free from grease. Unless this is the case uniform results are impossible. The liquid may be applied with a swab, or a brush, but if the object is small enough to dip into the liquid better results may thus be obtained than in any other way. The covering thus put on is said to be very lasting, and a sure protection against oxidation.
Blue.
I. Heat an iron bar to redness and lay it on a receptacle filled with water. On this bar place the objects to be blued, with the polished side up. As soon as the article has acquired the desired color cause it to fall quickly into the water. The pieces to be blued must always previously be polished with pumice stone or fine emery.
II. For screws: Take an old watch barrel and drill as many holes into the head of it as the number of screws to be blued. Fill it about one-fourth full of brass or iron filings, put in the head, and then fit a wire long enough to bend over for a handle, into the arbor holes head of the barrel upward. Brighten the heads of the screws, set them, point downward, into the holes already drilled, and expose the bottom of the barrel to the lamp, until the screws assume the color you wish.
III. To blue gun-barrels, etc., dissolve 2 parts of crystallized chloride of iron; 2 parts solid chloride of antimony; 1 part gallic acid in 4 or 5 parts of water; apply with a small sponge, and let dry in the air. Repeat this two or three times, then wash with water, and dry. Rub with boiled linseed oil to deepen the shade. Repeat this until satisfied with the result.
IV. The bluing of gun barrels is effected by heating evenly in a muffle until the desired blue color is raised, the barrel being first made clean and bright with emery cloth, leaving no marks of grease or dirt upon the metal when the bluing takes place, and then allow to cool in the air. It requires considerable experience to obtain an even clear blue.
Brown.
I. The following recipe for browning is from the United States Ordnance Manual: Spirits of wine, 1 1/2
[683]
STEEL
ounces; tincture of iron, 1 1/2 ounces; corrosive sublimate, 1 1/2 ounces; sweet spirits of niter, 1 1/2 ounces; blue vitriol, 1 ounce; nitric acid, 3/4 ounce. Mix and dissolve in 1 quart of warm water and keep in a glass jar. Clean the barrel well with caustic soda water to remove grease or oil. Then clean the surface of all stains and marks with emery paper or cloth, so as to produce an even, bright surface for the acid to act upon, and one without finger marks. Stop the bore and vent with wooden plugs. Then apply the mixture to every part with a sponge or rag, and expose to the air for 24 hours, when the loose rust should be rubbed off with a steel scratch brush. Use the mixture and the scratch brush twice, and more if necessary, and finally wash in boiling water, dry quickly, and wipe with linseed oil or varnish with shellac.
II. Apply four coats of the following solution, allowing each several hours to dry. Brush after each coat if necessary. After the last coat is dry, rub down hard.
Sulphate of copper 1 ounce
Sweet spirits of niter 1 ounce
Distilled water 1 pint
Niello. This is a brightly polished metal, which is provided with a black or blue-black foundation by heating, is covered with a design by the use of a suitable matrix and then treated with hydrochloric acid in such a manner that only the black ground is attacked, the metal underneath remaining untouched. Next, the acid is rinsed off and the reserve is removed with suitable solvents. The parts of the metal bared by the acid may also be provided with a galvanic coating of silver or other metal.
Another method is to plunge the articles for a few minutes into a solution of oxalic acid and to clean them by passing them through alcohol. In this way the polish can even be brought back without the use of rouge or diamantine.
Whitening or Blanching. If dissatisfied with the color acquired in tempering, dip the article into an acid bath, which whitens it, after which the bluing operation is repeated. This method is of great service, but it is important to remember always thoroughly to wash after the use of acid and then allow the object to remain for a few minutes in alcohol. Sulphuric acid does not whiten well, often leaving dark shades on the surface. Hydrochloric acid gives better results. Small pieces of steel are also whitened with a piece of pith moistened with dilute sulphuric acid, else the fine steel work, such as a watch hand, is fixed with lacquer on a plate and whitened by means of pith and polishing rouge, or a small stiff brush is charged with the same material. It is then detached by heating and cleaned in hot alcohol.
TEMPERING STEEL.
The best temperature at which to quench in the tempering of tool steel is the one just above the transformation point of the steel, and this temperature may be accurately determined in the following manner, without the use of a pyrometer. The pieces of steel are introduced successively at equal intervals of time into a muffle heated to a temperature a little above the transformation point of the steel. If, after a certain time, the pieces be taken out in the reverse order they will at first show progressively increasing degrees of brightness, these pieces being at the transformation point. When this point is passed the pieces again rapidly acquire a brightness superior to that of their neighbors, and should then be immediately quenched.
I. Heat red hot and dip in an unguent made of mercury and the fat of bacon. This produces a remarkable degree of hardness and the steel preserves its tenacity and an elasticity which cannot be obtained by other means.
II. Heat to the red white and thrust quickly into a stick of sealing wax. Leave it a second, and then change it to another place, and so continue until the metal is too cool to penetrate the wax. To pierce with drills hardened in this way, moisten them with essence of turpentine.
To Temper Small Coil Springs and Tools. To temper small coil springs in a furnace burning wood the springs are exposed to the heat of the flame and are quenched in a composition of the following preparation: To a barrel of fish oil, 10 quarts of rosin and 12 quarts of tallow are added. If the springs tempered in this mixture break, more tallow is added, but if the break indicates brittleness of the steel rather than excessive hardness, a ball of yellow beeswax about 6 inches in diameter is added. The springs are drawn to a reddish purple by being placed on a frame having horizontally radiating arms like a star which is mounted on the end of a vertical rod. The springs are laid on the star and are lowered into a pot of melted lead, being held there for such time as is required to draw to the desired color.
It is well known that the addition of
[684]
STEEL
certain soluble substances powerfully affects the action of tempering water. This action is strengthened if the heat conducting power of the water is raised by means of these substances; it is retarded if this power is reduced, or the boiling point substantially lowered. The substance most frequently used for the purpose of increasing the heat-conducting power of tempering water is common salt. This is dissolved in varying proportions of weight, a saturated solution being generally used as a quenching mixture. The use of this solution is always advisable when tools of complicated shape, for which a considerable degree of hardness is necessary, are to be tempered in large quantities or in frequent succession. In using these cooling fluids, care must be taken that a sufficient quantity is added to the water to prevent any great rise of temperature when the tempering process is protracted. For this reason the largest possible vessels should be used, wide and shallow, rather than narrow and deep, vessels being selected. Carbonate of soda and sal ammoniac do not increase the tempering action to the same extent as common salt, and are therefore not so frequently employed, though they form excellent additions to tempering water in certain cases. Tools of very complicated construction, such as fraises, where the danger of fracture of superficial parts has always to be kept in view, can with advantage be tempered in a solution of soda or sal ammoniac. Acids increase the action of tempering water considerably, and to a far greater extent than common salt. They are added in quantities up to 2 per cent, and frequently in combination with salts. Organic acids (e.g., acetic or citric) have a milder action than mineral acids (e.g., hydrochloric, nitric, or sulphuric). Acidulous water is employed in tempering tools for which the utmost degree of hardness is necessary, such as instruments for cutting exceptionally hard objects, or when a sufficiently hard surface has to be given to a kind of steel not capable of much hardening. Alcohol lowers the boiling point of water, and causes so vigorous an evaporation when the water comes in contact with the red hot metal, that the tempering is greatly retarded (in proportion to the amount of alcohol in the mixture). Water containing a large quantity of alcohol will not temper. Soap and soap suds will not temper steel; this property is made use of in the rapid cooling of steel for which a great degree of hardness is not desirable. When certain parts of completely tempered steel have to be rendered soft, these parts are heated to a red heat and then cooled in soap suds. This is done with the tangs of files, knives, swords, saws, etc. Soluble organic substances retard the tempering process in proportion to the quantity used, and thus lessen the effect of pure water. Such substances (e.g., milk, sour beer, etc.) are employed only to a limited extent.
To Caseharden Locally. In casehardening certain articles it is sometimes necessary, or desirable, to leave spots or sections in the original soft uncarbonized condition while the remainder is carbonized and hardened. This may be effected by first covering the parts to be hardened with a protecting coat of japan, and allowing it to dry. Then put the piece in an electroplating bath and deposit a heavy coat of nickel over the parts not protected by the japan. The piece thus prepared may be treated in the usual manner in casehardening. The coat of nickel prevents the metal beneath being carbonized, so it does not harden when dipped in the bath.
A plating of copper answers the same purpose as nickel and is often used. A simpler plan, where the shape of the piece permits, is to protect it from the action of the carbonizing material with an iron pipe or plate closely fitted or luted with clay. Another scheme is to machine the parts wanted soft after carbonizing but before hardening. By this procedure the 'carbonized material is removed where the metal is desired soft, and when heated and dipped these parts do not harden.
To Harden a Hammer. To avoid the danger of "checking" a hammer at the eye, heat the hammer to a good uniform hardening heat and then dip the small end almost up to the eye and cool as quickly as possible by moving about in the hardening bath; then dip the large end. To harden a hammer successfully by this method one must work quickly and cool the end dipped first enough to harden before the heat is lost on the other end. Draw the temper from the heat left about the eye. The result is a hammer hard only where it should be and free from "checks."
Hardening Steel Wire. Pass the steel wire through a lead bath heated to a temperature of 1,200 to 1,500º F. after it has previously been coated with a paste of chalk, so as to prevent the formation
[685]
STEEL
of oxides. The wire is thus heated in a uniform manner and, according to whether it is desired hard or elastic, it is cooled in water or in oil.
Hardening of Springs. A variety of steel must be chosen which is suitable for the production of springs, a very tough quality with about 0.8 per cent of carbon being probably the best. Any steel works of good reputation would no doubt recommend a certain kind of steel. In shaping a spring, forging and hammering should be avoided if possible. In forging, an uneven treatment can scarcely be avoided; one portion is worked more than the other, causing tensions which, especially in springs, must be guarded against. It is most advantageous if a material of the thickness and shape of the spring can be obtained, which, by bending and pressing through, is shaped into the desired spring. Since this also entails slight tension, a careful annealing is advisable, so as to prevent cracking or distorting in hardening. The annealing is best conducted with exclusion of the air, by placing the springs in a sheet-iron box provided with a cover, smearing all the joints well up with loam. The heating may be done in a muffled furnace; the box, with contents, is, not too slowly, heated to cherry red and then allowed to cool gradually, together with the stove. The springs must only be taken out when they have cooled off enough that they will give off no hissing sound when touched by water. In order to uniformly heat the springs for hardening, a muffle furnace is likewise employed, wherein they are heated to cherry-red heat. For cooling liquid, a mixture of oil, tallow, and petroleum is employed. A mass consisting of fish oil, tallow, and wax also renders good service, but one should see to it that there is a sufficient quantity of these cooling liquids, so that the springs may be moved about, same as when cooled in water, without causing an appreciable increase in the temperature of the liquid. In most cases too small a quantity of the liquid is responsible for the many failures in hardening. When the springs have cooled in the hardening liquid, they are taken out, dried off superficially, and the oil still adhering is burned off over a charcoal fire. This enables one to moderate the temper according to the duration of the burning off and to produce the desired elasticity. An even heating being of great importance in hardening springs, the electric current has of late been successfully employed for this purpose.
To Temper a Tap. After the tap has been cut and finished heat it in a pair of tongs to a blood-red heat over a charcoal fire or the blue flame of a Bunsen burner or blow pipe, turning it around so that one point does not get heated before another. Have ready a pail of clean, cold water, into which a handful of common salt has been put. Stir the water in the pail so that a whirlpool is set up. Then plunge the tap, point first and vertically, into the vortex to cool. The turning of the tap during heating, as well as the swirl of the quenching water, prevents distortion. In tempering, the temper of the tap requires to be drawn to a light straw color, and this may be done as follows: Get a piece of cast-iron tube about 3 inches in diameter and heat it to a dull-red heat for about 4 inches of its length. Then hold the tap, with the tongs, up the center of the tube, meanwhile turning the tap around until the straw color appears all over it. Then dip the tap in the water, when it will be found perfectly hard. The depth of the color, whether light or dark straw, must be determined by the nature of the cast steel being used, which can be gained only from experience of the steel.
Scissors Hardening. The united legs of the scissors are uniformly heated to a dark cherry red, extending from the point to the screw or rivet hole. This may be done in the naked fire, a feeble current of air being admitted until the steel commences to glow. Then the fire is left to itself and the scissor parts are drawn to and fro in the fire, until all the parts to be hardened show a uniform dark cherry red. The two legs are hardened together in water and then tempered purple red to violet.
The simultaneous heating, hardening, and tempering of the parts belonging together is necessary, so that the degree of heat is the same and the harder part does not cut the softer one.
In accordance with well-known rules, the immersion in the hardening bath should be done with the point first, slowly and vertically up to above the riveting hole.
Hardening without Scaling. Articles made of tool steel and polished may be hardened without raising a scale, thereby destroying the polish, by the following method: Prepare equal parts in bulk of common salt and (fine) corn meal, well mixed. Dip the article to be hardened first into water, then into the mixture and place it carefully into the fire. When hot enough to melt the mixture, take from
[686]
STEEL
the fire and dip or roll in the salt and meal, replace in the fire and bring to the required heat for hardening. Watch the piece closely and if any part of it shows signs of getting dry, sprinkle some of the mixture on it. The mixture, when exposed to heat, forms a flux over the surface of the steel which excludes the air and prevents oxidation, and when cooled in water or oil comes off easily, leaving the surface as smooth as before heating. Borax would possibly give the same result, but is sometimes difficult to remove when cold.
Hardening with Glycerine.
I. The glycerine employed must be of the density of 1.08 to 1.26 taken at the temperature of 302º F. Its weight must be equal to about 6 times the weight of the pieces to be tempered. For hard temper add to the glycerine 1/4 to 4 per cent of sulphate of potash or of manganese, and for soft temper 1 to 10 per cent of chloride of manganese, or 1 to 4 per cent of chloride of potassium. The temperature of the tempering bath is varied according to the results desired.
II. Glycerine, 8,000-parts, by weight; cooking salt, 500 parts, by weight; sal ammoniac, 100 parts, by weight; concentrated hydrochloric acid, 50 parts; and water, 10,000 parts, by weight. Into this liquid the steel, heated, for example, to a cherry red, is dipped. A reheating of the steel is not necessary.
To Remove Burnt Oil from Hardened Steel. To remove excess oil from parts that have been hardened in oil, place the articles in a small tank of gasoline, which, when exposed to the air, will dry off immediately, allowing the part to be polished and tempered without the confusing and unsightly marks of burnt oil.
VARIOUS RECIPES:
To Put an Edge on Steel Tools. Aluminum will put an edge on fine cutting instruments such as surgical knives, razors, etc. It acts exactly like a razor hone of the finest quality. When steel is rubbed on the aluminum, as, for instance, in honing a knife blade, the metal disintegrates, forming an infinitely minute powder of a greasy unctuous quality that clings to steel with great tenacity and thus assists in cutting away the surface of the harder metal. So fine is the edge produced that it can in no wise be made finer by the strop, which used in the ordinary way merely tends to round the edge.
To Restore Burnt Steel. To restore burnt cast steel heat the piece to a red heat and sprinkle over it a mixture of 8 parts red chromate of potassium; 4 parts saltpeter; 1/8 part aloes; 1/8 part gum arabic; and 1/4 part rosin.
To Remove Strains in Metal by Heating. In making springs of piano wire, or, in fact, any wire, if the metal is heated to a moderate degree the spring will be improved. Piano or any steel wire should be heated to a blue, brass wire to a degree sufficient to cause tallow to smoke. Heating makes the metal homogeneous; before heating, it is full of strains.
If a piece of metal of any kind is straightened cold and then put into a lathe and a chip turned off, it will be far from true. Before turning, it was held true by the strain of the particles on the outside, they having changed position, while the particles near the axis are only sprung. The outside particles being removed by the lathe tool, the sprung particles at the center return to their old positions. If, after straightening, the metal is heated to a temperature of 400º F., the particles settle together and the strains are removed.
This is the case in the manufacture of saws. The saw is first hardened and tempered and then straightened on an anvil by means of a hammer. After it is hammered true, it is ground and polished a little, then blued to stiffen it and then is subjected to the grinding process. Before bluing, the metal is full of strains; these are entirely removed by the heat required to produce the blue color. Often a piano-wire spring will not stand long wear if used without heating, while if heated it will last for years.
To Render Fine Cracks in Tools Visible. It is often of importance to recognize small cracks which appear in the metal of the tools. For this purpose it is recommended to moisten the fissured surface with petroleum; next rub and dry with a rag and rub again, but this time with chalk. The petroleum which has entered the cracks soon comes out again and the trace is plainly shown by the chalk.
To Utilize Drill Chips. There is one modern machining process that produces a shaving that has more value than that of mere scrap, and that is drilling rifle barrels with the oil-tube drill. The cutting edge of this drill is broken up into steps and the chips produced are literally shavings, being long hair-like threads of steel. These shavings are considerably used in woodworking factories for smoothing purposes.
[687]
STEEL
To Remove Fragments of Steel from Other Metals. The removal of broken spiral drills and taps is an operation which even the most skillful machinist has to perform at times. A practical process for removing such broken steel pieces consists in preparing in a suitable kettle (not iron) a solution of 1 part, by weight, of commercial alum in 4 to 5 parts, by weight, of water and boiling the object in this solution until the piece which is stuck works itself out. Care must be taken to place the piece in such a position that the evolving gas bubbles may rise and not adhere to the steel to protect it from the action of the alum solution.
Testing Steel. A bar of the steel to be tested is provided with about nine notches running around it in distances of about | of an inch. Next, the foremost notched piece is heated in a forge in such a manner that the remaining portion of the bar is heated less by the fire proper than by the transmitted heat. When the foremost piece is heated to burning, i.e, to combustion, and the color of the succeeding pieces gradually passes to dark-brownish redness, the whole rod is hardened. A test with the file will now show that the foremost burned piece possesses the greatest hardness, that several softer pieces will follow, and that again a piece ordinarily situated in the second third, whose temperature was the right one for hardening, is almost as hard as the first one. If the different pieces are knocked off, the fracture of the piece hardened at the correct temperature exhibits the finest grain. This will give one an idea of the temperature to be employed for hardening the steel in question and its behavior in general. Very hard steel will readily crack in this process.
Welding Compound. Boracic acid, 41 1/2 parts; common salt 35 parts; ferrocyanide of potassium, 20 parts; rosin, 7 1/2 parts; carbonate of sodium, 4 parts. Heat the pieces to be welded to a light red heat and apply the compound; then heat to a strong yellow heat and the welding may be accomplished in the usual manner.
The precaution should be observed, the same as with any of the cyanides, to avoid breathing the poisonous fumes.
Softening Steel. Heat the steel to a brown red and plunge into soft water, river water being the best. Care should be taken, however, not to heat over brown red, otherwise it will be hard when immersed. The steel will be soft enough to be cut with ease if it is plunged in the water as soon as it turns red.
Draw Tempering Cast Steel. First heat the steel lightly by means of charcoal until of a cherry-red shade, whereupon it is withdrawn to be put quickly into ashes or dry charcoal dust until completely cooled. The steel may also be heated in the forge to a red cherry color, then hammered until it turns blue and then plunged into water.
Drilling Hard Steel. To accomplish the object quickly, a drill of cast steel should be made, the point gradually heated to the red, the scales taken off, and the extremity of the point immersed at once in quicksilver; then the whole quenched in cold water. Thus prepared, the drill is equal to any emergency; it will bore through the hardest pieces. The quantity of quicksilver needed is trifling.
Engraving or Etching on Steel. Dissolve in 150 parts of vinegar, sulphate of copper, 30 parts; alum, 8 parts; kitchen salt, 11 parts. Add a few drops of nitric acid. According to whether this liquid is allowed to act a longer or shorter time, the steel may be engraved upon deeply or the surface may be given a very ornamental, frosted appearance.
To Distinguish Steel from Iron. Take a very clean file and file over the flame of an alcohol lamp. If the filed piece is made of steel, little burning and crackling sparks will be seen. If it consists of iron, the sparks will not crackle.
STEEL, BROWNING OF:
See Plating.
STEEL, DISTINGUISHING IRON FROM:
See Iron.
STEEL ETCHING:
See Etching.
STEEL-HARDENING POWDER:
See Iron.
STEEL, OXIDIZED:
See Plating.
STEEL PLATING:
See Plating.
STEEL POLISHES:
See Polishes.
STEEL, TO CLEAN:
See Cleaning Preparations and Methods.
[688]
STEREOCHROMY - STONE
STENCILS FOR PLOTTING LETTERS OF SIGN PLATES:
See Enameling.
STENCIL INKS:
See Inks.
STEREOCHROMY.
Stereochromatic colors can be bought ground in a thickly liquid water-glass solution. They are only diluted with water-glass solution before application on the walls. The two solutions are generally slightly dissimilar in their composition, the former containing less silicic acid, but more alkali, than the latter, which is necessary for the better preservation of the paint. Suitable pigments are zinc white, ocher with its different shades of light yellow, red, and dark brown, black consisting of a mixture of manganese and lampblack, etc., etc. White lead cannot be used, as it coagulates with the water glass, nor vermilion, because it fades greatly under the action of the light. The plastering to be coated must be porous, not fresh, but somewhat hardened. Otherwise the caustic lime of the plaster will quickly decompose the water glass. This circumstance may account for the unsatisfactory results which have frequently been obtained with water-glass coatings. Before applying the paint the wall should first be impregnated with a water-glass solution. The colors may be kept on hand ground, but must be protected from contact with the air. If air is admitted a partial separation of silica in the form of a jelly takes place. Only pure potash water glass, or, at least, such as only contains little soda, should be used, as soda will cause efflorescence.
STEREOPTICON SLIDES:
See Photography.
STEREOTYPE METAL:
See Alloys.
STONE, ARTIFICIAL.
The following is a process of manufacture in which the alkaline silicates prepared industrially are employed.
The function of the alkaline silicates, or soluble glass, as constituents of artificial stone, is to act as a cement, forming with the alkaline earths, alumina, and oxide of lead, insoluble silicates, which weld together the materials (quartz sand, pebbles, granite, fluorspar, and the waste of clay bricks). The mass may be colored black by the addition of a quantity of charcoal or graphite to the extent of 10 per cent at the maximum, binoxide of manganese, or ocher; red, by 6 per cent of colcothar; brick red, by 4 to 7 per cent of cinnabar; orange, by 6 to 8 per cent of red lead; yellow, by 6 per cent of yellow ocher, or 5 per cent of chrome yellow; green, by 8 per cent of chrome green; blue, by 6 to 10 per cent of Neuwied blue, Bremen blue, Cassel blue, or Napoleon blue; and white, by 20 per cent, at the maximum, of zinc white.
Chrome green and zinc oxide produce an imitation of malachite. An imitation of lapis lazuli is obtained by the simultaneous employment of Cassel blue and pyrites in grains. The metallic oxides yield the corresponding silicates, and zinc oxide, mixed with cleansed chalk, yields a brilliant marble. The ingredients are mixed in a kind of mechanical kneading trough, furnished with stirrers, in variable proportions, according to the percentage of the solution of alkaline silicate. The whole is afterwards molded or compressed by the ordinary processes.
The imitation of granite is obtained by mixing lime, 100 parts; sodium silicate (42º Bé.), 35 parts; fine quartz sand, 120 to 180 parts; and coarse sand, 180 to 250 parts..
Artificial basalt may be prepared by adding potassium sulphite and lead acetate, or equal parts of antimony ore and iron filings.
To obtain artificial marble, 100 pounds of marble dust or levigated chalk are mixed with 20 parts of ground glass and 8 parts of fine lime and sodium silicate. The coloring matter is mixed in proportion depending on the effect to be produced.
A fine product for molding is obtained by mixing alkaline silicate, 100 parts; washed chalk, 100 parts; slaked lime, 40 parts; quick lime, 40 parts, fine quartz sand, 200 parts; pounded glass, 80 parts; infusorial earths, 80 parts; fluorspar, 150 parts. On hardening, there is much contraction.
Other kinds of artificial stone are prepared by mixing hydraulic lime or cement, 50 parts; sand, 200 parts; sodium silicate, in dry powder, 50 parts; the whole is moistened with 10 per cent of water and molded.
A hydraulic cement may be employed, to which an alkaline silicate is added. The stone or object molded ought to be covered with a layer of fluosilicate.
[689]
STONE
A weather-proof water-resisting stone is manufactured from sea mud, to which 5 per cent of calcic hydrate is added. The mass is then dried, lixiviated, and dried once more at 212º F., whereupon the stones are burned. By an admixture of crystallized iron sulphate the firmness of these stones is still increased.
Sand Lime Brick. In a French patent for making bricks from pitch and coal tar, powdered coke and sea sand are gently heated in a suitable vessel, and 20 per cent of pitch and 10 per cent of coal tar added, with stirring. The pasty mass obtained is then molded under pressure. The product obtained may be employed alone, or together with a framework of iron, or with hydraulic lime or cement.
According to a French patent for veining marble, etc., in one or more colors, coloring matters of all kinds are mixed with a sticky liquid, which is then spread in a very thin layer on the surface of another immiscible and heavier liquid. By agitating the surface, colored veins, etc., are obtained, which are then transferred to the object to be decorated (which may be of most varied kind) by applying it to the surface of the heavy liquid. A suitable composition with which tfye colors may be mixed consists of: Oil of turpentine, 100 parts; colophony, 10 parts; linseed oil, 10 parts; siccatif soleil, 5 parts. The heavy liquid may be water, mercury, etc.; and any colors, organic or mineral, may be used.
CONCRETE.
Concrete is the name applied to an artificial combination of various mineral substances which under chemical action become incorporated into a solid mass. There are one or two compositions of comparatively trifling importance which receive the same name, though differing fundamentally from true concrete, their solidification being independent of chemical influence. These compositions only call for passing mention; they are: Tar concrete, made of broken stones (macadam) and tar; iron concrete, composed of iron turnings, asphalt, bitumen, and pitch; and lead concrete, consisting of broken bricks set in molten lead. The last two varieties, with rare exceptions, are only used in connection with military engineering, such as for fortifications.
Concrete proper consists essentially of two groups or classes of ingredients. The first, termed the aggregate, is a heterogeneous mass, in itself inactive, of mineral material, such as shingle, broken stone, broken brick, gravel, and sand. These are the substances most commonly in evidence, but other ingredients are also occasionally employed, such as slag from iron furnaces. Burnt clay, in any form, and earthenware, make admirable material for incorporation. The second class constitutes the active agency which produces adhesion and solidification. It is termed the matrix, and consists of hydraulic lime or cement, combined with water.
One of the essential features in good concrete is cleanliness and an entire absence of dirt, dust, greasy matter, and impurities of any description. The material will preferably be sharp and angular, with a rough, porous surface, to which the matrix will more readily adhere than to smooth, vitreous substances. The specific gravity of the aggregate will depend upon the purpose for which the concrete is to be used. For beams and lintels, a light aggregate, such as coke breeze from gasworks, is permissible, especially when the work is designed to receive nails. On the other hand, for retaining walls, the heaviest possible aggregate is desirable on the ground of stability.
The aggregate by no means should be uniform in size. Fragments of different dimensions are most essential, so that the smaller material may fill up the interstices of the larger. It is not infrequently stipulated by engineers that no individual fragment shall be more than 4 inches across, and the material is often specified to pass through a ring 1 1/2 to 2 inches in diameter. The absolute limits to size for the aggregate, however, are determinable by a number of considerations, not the least important of which is the magnitude and bulk of the work in which it is to be employed. The particles of sand should also be of varying degrees of coarseness. A fine, dustlike sand is objectionable; its minute subdivision prevents complete contact with the cement on all its faces. Another desideratum is that the particles should not be too spherical, a condition brought about by continued attrition. Hence, pit sand is better in many cases than river sand or shore sand.
The matrix is almost universally Portland cement. It should not be used in too hot a condition, to which end it is usually spread over a wooden floor to a depth of a few inches, for a few days prior to use. By this means, the aluminate of lime becomes partially hydrated, and its activity is thereby modified,
[690]
STONE
Roman cement and hydraulic lime may also be used as matrices.
Portland cement will take a larger proportion of sand than either Roman cement or hydraulic lime; but with the larger ratios of sand, its tenacity is, of course, correspondingly reduced. One part of cement to 4 parts of sand should therefore be looked upon as the upper limit, while for the strongest mortar the proportion need hardly exceed 1 part of cement to 1 1/2 or 2 parts of sand. In the ensuing calculations there is assumed a ratio of 1 to 3. For impermeability, the proportion of 1 to 2 should be observed, and for Roman cement this proportion should never be exceeded. The ratio will even advantageously be limited to 2 to 3. For hydraulic lime equal parts of sand and cement are suitable, though 2 parts of sand to 1 part of cement may be used.
The quantity of mortar required in reference to the aggregate is based on the vacuities in the latter. For any particular aggregate the amount of empty space may be determined by filling a tank of known volume with the minerals and then adding sufficient water to bring to a level surface. The volume of water added (provided, of course, the aggregate be impervious or previously saturated) gives the net volume of mortar required. To this it is necessary to make some addition (say 10 per cent of the whole), in order to insure the thorough flushing of every part of the work.
Assuming that the proportion of interstices is 30 per cent and adding 10 for the reason just stated, we derive 40 parts as the quantity of mortar to 100 - 10 = 90 parts of the aggregate. An allowance of 1/4 volume for shrinkage brings the volume of the dry materials (sand and cement) of the mortar to 40 + 40/3 = 53 1/3 parts, which, divided in the ratio of 1 to 3, yields:
53 1/3
Cement --------- = 13 1/2 parts
4
Sand, ¾ X 53 1/3 = 40 parts
Aggregate 90 parts
Total 143 1/3 parts
As the resultant concrete is 100 parts, the total shrinkage is 30 per cent. Expressed in terms of the cement, the concrete would have a composition of 1 part cement, 3 parts sand, 7 parts gravel and broken stone, and it would form, approximately, what is commonly known as 7 to 1 concrete.
There are other ratios depending on the proportion of sand. Thus we have:
Cement Sand Aggregate
1 1 1/2 4 1/3
1 2 5
1 2 1/2 6
1 3 7
1 2 1/2 7 1/2
1 4 8 1/4
The cost of concrete may be materially reduced without affecting the strength or efficacy of the work, by a plentiful use of stone "plums" or "burrs." These are bedded in the fluid concrete during its deposition in situ, but care must be taken to see that they are thoroughly surrounded by mortar and not in contact with each other. Furthermore, if they are of a porous nature, they should be well wetted before use.
The mixing of concrete is important. If done by hand, the materials forming the aggregate will be laid out on a platform and covered by the cement in a thin layer. The whole should be turned over thrice in the dry state, and as many times wet, before depositing, in order to bring about thorough and complete amalgamation. Once mixed, the concrete is to be deposited immediately and allowed to remain undisturbed until the action of setting is finished. Deposition should be effected, wherever possible, without tipping from a height of more than about 6 feet, as in greater falls there is a likelihood of the heavier portions of the aggregate separating from the lighter. In extensive undertakings, concrete is more economically mixed by mechanical appliances.
The water used for mixing may be either salt or fresh, so far as the strength of the concrete is concerned. For surface work above the ground level, salinity in any of the ingredients is objectionable, since it tends to produce efflorescence an unsightly, floury deposit, difficult to get rid of. The quantity of water required cannot be stated with exactitude; it will depend upon the proportion of the aggregate and its porosity. It is best determined by experiment in each particular case. Without being profuse enough to "drown" the concrete, it should be plentiful enough to act as an efficient intermediary between every particle of the aggregate and every particle of the matrix. Insufficient moisture is, in fact, as deleterious as an excess.
Voids. The strength of concrete depends greatly upon its density, and this is secured by using coarse material which contains the smallest amount of voids or empty spaces. Different kinds of sand,
[691]
STONE
gravel, and stone vary greatly in the amount of voids they contain, and by judiciously mixing coarse and fine material the voids may be much reduced and the density increased. The density and percentage of voids in concrete material may be determined by filling a box of 1 cubic foot capacity and weighing it. One cubic foot of solid quartz or limestone, entirely free from voids, would weigh 165 pounds, and the amount by which a cubic foot of any loose material falls short of this weight represents the proportion of voids contained in it. For example, if a cubic foot of sand weighs 115 1/2 pounds, the voids would be 49 1/2-165ths of the total volume, or 30 per cent.
The following table gives the per cent of voids and weight per cubic foot of some common concrete materials:
Per Cent Voids Wt. per Cu. Ft.
Sandusky Bay sand 32. 3 111.7 pounds
Same through 20 mesh screen 38.5 101.5 pounds
Gravel, 1/8 to 1/4 inch 42.4 95.0 pounds
Broken limestone, egg-size 47.0 87.4 pounds
Limestone screenings,
dust to 1/2 inch 26.0 122.2 pounds
It will be noted that screening the sand through a 20-mesh sieve, and thus taking out the coarse grains, considerably increased the voids and reduced the weight; thus decidedly injuring the sand for making concrete.
The following figures show how weight can be increased and voids reduced by mixing fine and coarse material:
Per Cent Voids Wt. per Cu. Ft.
Pebbles, about 1 inch 38.7 101.2 pounds
Sand, 30 to 40 mesh 35.9 105.8 pounds
Pebbles plus 38.7 per
cent sand, by vol. 19.2 133.5 pounds
Experiments have shown that the strength of concrete increases greatly with its density; in fact, a slight increase in weight per cubic foot adds very decidedly to the strength.
The gain in strength obtained by adding coarse material to mixtures of cement and sand is shown in the following table of results of experiments made in Germany by R. Dykerhoff. The blocks tested were 2 1/2 inch cubes, 1 day in air and 27 days in water,
|
Proportions by Measure.
|
Per Cent Cement.
|
Compression Strength.
|
||
|
Cement.
|
Sand.
|
Gravel.
|
By Volume.
|
Lbs. per Sq. In.
|
|
1 1 1 1 1 1 |
2 2 3 3 4 4 |
... 5 ... 6 1/2 ... 8 1/2 |
33.0 12.5 25.0 9.5 20.0 7.4 |
2,125 2.387 1,383 1,515 1,053 1,204 |
These figures show how greatly the strength is improved by adding coarse material, even though the proportion of cement is thereby reduced. A mixture of 1 to 12 1/2 of properly proportioned sand and gravel is, in fact, stronger than 1 to 4, and nearly as strong as 1 to 3, of cement and sand only.
In selecting materials for concrete, those should be chosen which give the greatest density. If it is practicable to mix two materials, as sand and gravel, the proportion which gives the greatest density should be determined by experiment, and rigidly adhered to in making concrete, whatever proportion of cement it is decided to use. Well-proportioned dry sand and gravel or sand and broken stone, well shaken down, should weigh at least 125 pounds per cubic foot. Limestone screenings, owing to minute pores in the stone itself, are somewhat lighter, though giving equally strong concrete. They should weigh at least 120 pounds per cubic foot. If the weight is less, there is probably too much fine dust in the mixture.
The density and strength of concrete are also greatly improved by use of a liberal amount of water. Enough water must be used to make the concrete thoroughly soft and plastic, so as to quake strongly when rammed. If mixed too dry it will never harden properly, and will be light, porous, and crumbling.
Thorough mixing of concrete materials is essential, to increase the density and give the cement used a chance to produce its full strength. The cement, sand, and gravel should be intimately mixed dry, then the water added and the mixing continued. If stone or coarse gravel is added, this should be well wetted and thoroughly mixed with the mortar.
Materials for Concrete Building Blocks. In the making of building blocks the spaces to be filled with concrete are generally too narrow to permit the use of very coarse material, and the block
[692]
STONE
maker is limited to gravel or stone not exceeding 1/2 or 3/4 inch in size. A considerable proportion of coarse material is, however, just as necessary as in other kinds of concrete work, and gravel or screenings should be chosen which will give the greatest possible density. For good results, at least one-third of the material, by weight, should be coarser than 1/8 inch. Blocks made from such gravel or screenings, 1 to 5, will be found as good as 1 to 3 with sand only. It is a mistake to suppose that the coarse fragments will snow on the surface; if the mixing is thorough this will not be the case. A moderate degree of roughness or variety in the surface of blocks is, in fact, desirable, and would go far to overcome the prejudice which many architects hold against the smooth, lifeless surface of cement work. Sand and gravel are, in most cases, the cheapest material to use for block work. The presence of a few per cent of clay or loam is not harmful provided the mixing is thorough. Stone screenings, if of good quality, give fully as strong concrete as sand and gravel, and usually yield blocks of somewhat lighter color. Screenings from soft stone should be avoided, also such as contain too much dust. This can be determined from the weight per cubic foot, and by a sifting test. If more than two-thirds pass 1/8 inch, and the weight (well jarred down) is less than 120 pounds, the material is not the best. Cinders are sometimes used for block work; they vary greatly in quality, but if clean and of medium coarseness will give fair results. Cinder concrete never develops great strength, owing to the porous character and crushability of the cinders themselves. Cinder blocks may, however, be strong enough for many purposes, and suitable for work in which great strength is not required.
Lime. It is well known that slaked lime is a valuable addition to cement mortar, especially for use in air. In sand mixtures, 1 to 4 or 1 to 5, at least one-third of the cement may be replaced by slaked lime without loss of strength. The most convenient form of lime for use in block making is the dry-slaked or hydrate lime, now a common article of commerce. This is, however, about as expensive as Portland cement, and there is no great saving in its use. Added to block concrete, in the proportion of 1/4 to 1/2 the cement used, it will be found to make the blocks lighter in color, denser, and decidedly less permeable by water.
Cement. Portland cement is the only hydraulic material to be seriously considered by the block maker. Natural and slag cements and hydraulic lime are useful for work which remains constantly wet, but greatly inferior in strength and durability when exposed to dry air. A further advantage of Portland cement is the promptness with which it hardens and develops its full strength; this quality alone is sufficient to put all other cements out of consideration for block work.
Proportions. There are three important considerations to be kept in view in adjusting the proportions of materials for block concrete strength, permeability, and cost. So far as strength goes, it may easily be shown that concretes very poor in cement, as 1 to 8 or 1 to 10, will have a crushing resistance far beyond any load that they may be called upon to sustain. Such concretes are, however, extremely porous, and absorb water like a sponge. The blocks must bear a certain amount of rough handling at the factory and while being carted to work and set up in the wall. Safety in this respect calls for a much greater degree of hardness than would be needed to bear the weight of the building. Again, strength and hardness, with a given proportion of cement, depend greatly on the character of the other materials used; blocks made of cement and sand, 1 to 3, will not be so strong or so impermeable to water as those made from a good mixed sand and gravel, 1 to 5. On the whole, it is doubtful whether blocks of satisfactory quality can be made, by hand mixing and tamping, under ordinary factory conditions, from a poorer mixture than 1 to 5. Even this proportion requires for good results the use of properly graded sand and gravel or screenings, a liberal amount of water, and thorough mixing and tamping. When suitable gravel is not obtainable, and coarse mixed sand only is used, the proportion should not be less than 1 to 4. Fine sand alone is a very bad material, and good blocks cannot be made from it except by the use of an amount of cement which would make the cost very high.
The mixtures above recommended, 1 to 4 and 1 to 5, will necessarily be somewhat porous, and may be decidedly so if the gravel or screenings used is not properly graded. The water-resisting qua.ities may be greatly improved, without loss of strength, by replacing a part of the cement by hydrate lime. This is a light, extremely fine material, and a given weight of it goes much further than the
[693]
STONE
same amount of cement in filling the pores of the concrete. It has also the effect of making the wet mixture more plastic and more easily compacted by ramming, and gives the finished blocks a lighter color.
The following mixtures, then, are to be recommended for concrete blocks. By "gravel" is meant a suitable mixture of sand and gravel, or stone screenings, containing grains of all sizes, from fine to 1/2 inch.
1 to 4 Mixtures, by Weight.
Cement, 150 parts; gravel, 600 parts.
Cement, 125 parts; hydrated lime, 25 parts; gravel, 600 parts.
Cement, 100 parts; hydrated lime, 50 parts; gravel, 600 parts.
1 to 5 Mixtures, by Weight.
Cement, 120 parts; gravel, 600 parts.
Cement, 100 parts; hydrated lime, 20 parts; gravel, 600 parts.
Proportion of Water. This is a matter of the utmost consequence, and has more effect on the quality of the work than is generally supposed. Blocks made from too dry concrete will always remain soft and weak, no matter how thoroughly sprinkled afterwards. On the other hand, if blocks are to be removed from the machine as soon as made, too much water will cause them to stick to the plates and sag out of shape. It is perfectly possible, however, to give the concrete enough water for maximum density and first-class hardening properties, and still to remove the blocks at once from the mold. A good proportion of coarse material allows the mixture to be made wetter without sticking or sagging. Use of plenty of water vastly improves the strength, hardness, and waterproof qualities of blocks, and makes them decidedly lighter in color. The rule should be:
Use as much water as possible without causing the blocks to stick to the plates or to sag out of shape on removing from the machine.
The amount of water required to produce this result varies with the materials used, but is generally from 8 to 9 per cent of the weight of the dry mixture. A practiced block maker can judge closely when the right amount of water has been added, by squeezing some of the mixture in the hand. Very slight variations in proportion of water make such a marked difference in the quality and color of the blocks that the water, when the proper quantity for the materials used has been determined, should always be accurately measured out for each batch. In this way much time is saved and uncertainty avoided.
Facing. Some block makers put on a facing of richer and finer mixture, making the body of the block of poorer and coarser material. As will be explained later, the advantage of the practice is, in most cases, questionable, but facings may serve a good purpose in case a colored or specially waterproof surface is required. Facings are generally made of cement and sand, or fine screenings, passing a 1/8 inch sieve. To get the same hardness and strength as a 1 to 5 gravel mixture, at least as rich a facing as 1 to 3 will be found necessary. Probably 1 to 2 will be found better, and if one third the cement be replaced by hydrate lime the waterproof qualities and appearance of the blocks will be improved. A richer facing than 1 to 2 is liable to show greater shrinkage than the body of the block, and to adhere imperfectly or develop hair-cracks in consequence.
Poured Work. The above suggestions on the question of proportions of cement, sand, and gravel for tamped blocks apply equally to concrete made very wet, poured into the mold, and allowed to harden a day or longer before removing. Castings in a sand mold are made by the use of very liquid concrete; sand and gravel settle out too rapidly from such thin mixtures, and rather fine limestone screenings are generally used.
Mixing. To get the full benefit of the cement used it is necessary that all the materials shall be very thoroughly mixed together. The strength of the block as a whole will be only as great as that of its weakest part, and it is the height of folly, after putting a liberal measure of cement, to so slight the mixing as to get no better result than half as much cement, properly mixed, would have given. The poor, shoddy, and crumbly blocks turned put by many small-scale makers owe their faults chiefly to careless mixing and use of too little water, rather than to too small proportion of cement.
The materials should be mixed dry, until the cement is uniformly distributed and perfectly mingled with the sand and gravel or screenings; then the water is to be added and the mixing continued until all parts of the mass are equally moist and every particle is coated with the cement paste.
Concrete Mixers. Hand mixing is always imperfect, laborious, and slow.
[694]
STONE
and it is impossible by this method to secure the thorough stirring and kneading action which a good mixing machine gives. If a machine taking 5 or 10 horse-power requires 5 minutes to mix one-third of a yard of concrete, it is of course absurd to expect that two men will do the same work by hand in the same time. And the machine never gets tired or shirks if not constantly urged, as it is the nature of men to do. It is hard to see how the manufacture of concrete blocks can be successfully carried on without a concrete mixer. Even for a small business it will pay well in economy of labor and excellence of work to install such a machine, which may be driven by a small electric motor or gasoline engine. In work necessarily so exact as this, requiring perfectly uniform mixtures and use of a constant percentage of water, batch mixers, which take a measured quantity of material, mix it, and discharge it, at each operation, are the only satisfactory type, and continuous mixers are unsuitable. Those of the pug mill type, consisting of an open trough with revolving paddles and bottom discharge, are positive and thorough in their action, and permit the whole operation to be watched and controlled. They should be provided with extensible arms of chilled iron, which can be lengthened as the ends become worn.
Concrete Block Systems. For smaller and less costly buildings, separate blocks, made at the factory and built up into the walls in the same manner as brick or blocks of stone, are simpler, less expensive, and much more rapid in construction than monolithic work. They also avoid some of the faults to which solid concrete work, unless skillfully done, is subject, such as the formation of shrinkage cracks.
There are two systems of block making, differing in the consistency of the concrete used:
1. Blocks tamped or pressed from semi-wet concrete, and removed at once from the mold.
2. Blocks poured or tamped from wet concrete, and allowed to remain in the mold until hardened.
Tamped Blocks from Semi-Wet Mixture. These are practically always made on a block machine, so arranged that as soon as a block is formed the cores and side plates are removed and the block lifted from the machine. By far the larger part of the blocks on the market are made in this way. Usually these are of the one-piece type, in which a single block, provided with hollow cores, makes the whole thickness of the wall. Another plan is the two-piece system, in which the face and back of the wall are made up of different blocks, so lapping over each other as to give a bond and hold the wall together. Blocks of the two-piece type are generally formed in a hand or hydraulic press.
Various shapes and sizes of blocks are commonly made; the builders of the most popular machines have, however, adopted the standard length of 32 inches and height of 9 inches for the full-sized block, with thickness of 8, 10, and 12 inches. Lengths of 24, 16, and 8 inches are also obtained on the same machines by the use of parting plates and suitably divided face plates; any intermediate lengths and any desired heights may be produced by simple adjustments or blocking off.
Blocks are commonly made plain, rock-faced, tool-faced, paneled, and of various ornamental patterns. New designs of face plates are constantly being added by the most progressive machine makers.
Block Machines. There are many good machines on the market, most of which are of the same general type, and differ only in mechanical details. They may be divided into two classes: those with vertical and those with horizontal face. In the former the face plate stands vertically, and the block is simply lifted from the machine on its base plate as soon as tamped. In the other type the face plate forms the bottom of the mold; the cores are withdrawn horizontally, and by the motion of a lever the block with its face plate is tipped up into a vertical position for removal. In case it is desired to put a facing on the blocks, machines of the horizontal-face type are considered the more convenient, though a facing may easily be put on with the vertical-face machine by the use of a parting plate.
Blocks Poured from Wet Concrete. As already stated, concrete made too dry is practically worthless, and an excess of water is better than a deficiency. The above-described machine process, in which blocks are tamped from damp concrete and at once removed, gives blocks of admirable hardness and quality if the maximum of water is used. A method of making blocks from very wet concrete, by the use of a large number of separable molds of sheet steel, into which the wet concrete is poured and in which the blocks are left to harden for 24
[695]
STONE
hours or longer, has come into considerable use. By this method blocks of excellent hardening and resistance to water are certainly obtained. Whether the process is the equal of the ordinary machine method in respect of economy and beauty of product must be left to the decision of those who have had actual experience with it.
The well known cast stone process consists in pouring liquid concrete mixture into a sand mold made from a pattern in a manner similar to that in which molds for iron castings are produced. The sand absorbs the surplus water from the liquid mixture, and the casting is left in the mold for 24 hours or longer until thoroughly set. This process necessitates the making of a new sand mold for every casting, and is necessarily much less rapid than the machine method. It is less extensively used for building blocks than for special ornamental architectural work, sills, lintels, columns, capitals, etc., and for purposes of this kind it turns out products of the highest quality and beauty.
Tamping of Concrete Blocks. This is generally done by means of hand rammers. Pneumatic tampers, operated by an air compressor, are in use at a few plants, apparently with considerable saving in time and labor and improvements in quality of work. Hand tamping must be conscientious and thorough, or poor work will result. It is important that the mold should be filled a little at a time, tamping after each addition; at least four fillings and tampings should be given to each block. If the mixture is wet enough no noticeable layers will be formed by this process.
Hardening and Storage. Triple decked cars to receive the blocks from the machines will be found a great saving of labor, and are essential in factories of considerable size. Blocks will generally require to be left on the plates for at least 24 hours, and must then be kept under roof, in a well-warmed room, with frequent sprinkling, for not less than 5 days more. They may then be piled up out of doors, and in dry weather should be wetted daily with a hose. Alternate wetting and drying is especially favorable for the hardening of cement, and concrete so treated gains much greater strength than if kept continuously in water or dry air.
Blocks should not be used in building until at least 4 weeks from the time they are made. During this period of seasoning, blocks will be found to shrink at least 1/16 inch in length, and if built up in a wall when freshly made, shrinkage cracks in the joints or across the blocks will surely appear.
Efflorescence, or the appearance of a white coating on the surfaces, sometimes takes place when blocks are repeatedly saturated with water and then dried out; blocks laid on the ground are more liable to show this defect. It results from diffusion of soluble sulphates of lime and alkalies to the surface. It tends to disappear in time, and rarely is sufficient in amount to cause any complaint.
Properties of Concrete Blocks Strength. In the use of concrete blocks for the walls of buildings, the stress to which they are subjected is almost entirely one of compression. In compressive strength well-made concrete does not differ greatly from ordinary building stone. It is difficult to find reliable records of tests of sand and gravel concrete, 1 to 4 and 1 to 5, such as is used in making blocks; the following figures show strength of concrete of approximately this richness, also the average of several samples each of well-known building stones, as stated by the authorities named:
Limestone, Bedford, Ind.
(Indiana Geographical Survey) 7,792 pounds
Limestone, Marblehead, Ohio
(Q. A. Gillmore) 7,393 pounds
Sandstone, N. Amherst, Ohio
(Q. A. Gillmore) 5,831 pounds
Gravel concrete, 1:1.6:2.8, at 1 year
(Candlot) 5,500 pounds
Gravel concrete, 1:1.6:3.7, at 1 year
(Candlot) 5,050 pounds
Stone concrete, 1:2:4 at 1 year
(Boston El. R.R.) 3,904 pounds
Actual tests of compression strength of hollow concrete blocks are difficult to make, because it is almost impossible to apply the load uniformly over the whole surface, and also because a block 16 inches long and 8 inches wide will bear a load of 150,000 to 200,000 pounds, or more than the capacity of any but the largest testing machines. Three one quarter blocks, 8 inches long, 8 inches wide, and 9 inches high, with hollow space equal to one-third of the surface, tested at the Case School of Science, showed strengths of 1,805, 2,000, and.
[696]
STONE
1,530 pounds per square inch, respectively, when 10 weeks old.
Two blocks 6 X 8 X 9 inches, 22 months old, showed crushing strength of 2,530 and 2,610 pounds per square inch. These blocks were made of cement 1 1/4 parts, lime 1/2 part, sand and gravel 6 parts, and were tamped from damp mixture. It is probably safe to assume that the minimum crushing strength of well-made blocks, 1 to 5, is 1,000 pounds per square inch at 1 month and 2,000 pounds at 1 year.
A block 12 inches wide and 24 inches long has a total surface of 288 square inches, or, deducting 1/3 for openings, a net area of 192 inches. Such a block, 9 inches high, weighs 130 pounds. Assuming a strength of 1,000 pounds and a factor of safety of 5, the safe load would be 200 pounds per square inch, or 200 X 192 = 38,400 pounds for the whole surface of the block. Dividing this by the weight of the block, 130 pounds, we find that 295 such blocks could be placed one upon another, making a total height of wall of 222 feet, and still the pressure on the lowest block would be less than one-fifth of what it would actually bear. This shows how greatly the strength of concrete blocks exceeds any demands that are ever made upon it in ordinary building construction.
The safe load above assumed, 200 pounds, seems low enough to guard against any possible failure. In Taylor and Thompson's work on concrete, a safe load of 450 pounds for concrete 1 to 2 to 4 is recommended; this allows a factor of safety of 5 1/2. On the other hand, the Building Code of the city of Cleveland permits concrete to be loaded only to 150 pounds per square inch, and limits the height of walls of 12 inch blocks to 44 feet. The pressure of such a wall would be only 40 pounds per square inch; adding the weight of two floors at 25 pounds per square foot each, and roof with snow and wind pressure, 40 pounds per square foot, we find that with a span of 25 feet the total weight on the lowest blocks would be only 52 pounds per square inch, or about one twentieth of their minimum compression strength.
Blocks with openings equal to only one third the surface, as required in many city regulations, are heavy to handle, especially for walls 12 inches and more in thickness, and, as the above figures show, are enormously stronger than there is any need of. Blocks with openings of 50 per cent would be far more acceptable to the building trade, and if used in walls not over 44 feet high, with floors and roof calculated as above for 25 feet span, would be loaded only to 56 pounds per square inch of actual surface. This would give a factor of safety of 18, assuming a minimum compression strength of 1,000 pounds.
There is no doubt that blocks with one-third opening are inconveniently and unnecessarily heavy. Such a block, 32 inches long, 12 inches wide, and 9 inches high, has walls about 3 1/2 inches thick, and weighs 180 pounds. A block with 50 per cent open space would have walls and partitions 2 inches in thickness, and would weigh about 130 pounds. With proper care in manufacture, especially by using as much water as possible, blocks with this thickness of walls may be made thoroughly strong, sound, and durable. It is certainly better for strength and water-resisting qualities to make thin walled blocks of rich mixture, rather than heavy blocks of poor and porous material.
Filling the voids with cement is a rather expensive method of securing waterproof qualities, and gives stronger concretes than are needed. The same may be accomplished more cheaply by replacing part of the cement by slaked lime, which is an extremely fine-grained material, and therefore very effective in closing pores. Hydrate lime is the most convenient material to use, but nearly as costly as Portland cement at present prices. A 1 to 4 mixture in which one-third the cement is replaced by hydrate lime will be found equal to a 1 to 3 mixture without the lime. A 1 to 4 concrete made from cement, 1; hydrate lime, 1/2; sand and gravel, 6 (by weight), will be found fairly water-tight, and much superior in this respect to one of the same richness consisting of cement, 1 1/2; sand and gravel, 6.
The cost of lime may be greatly reduced by using ordinary lump lime slaked to a paste. The lime must, however, be very thoroughly hydrated, so that no unslaked fragments may remain to make trouble by subsequent expansion. Lime paste is also very difficult to mix, and can be used successfully only in a concrete mixer of the pug mill type. Ordinary stiff lime paste contains about 50 per cent water; twice as much of it, by weight, should therefore be used as of dry hydrate lime.
Waterproof Qualities. The chief fault of concrete building blocks, as ordinarily made, is their tendency to absorb water. In this respect they are generally no
[697]
STONE
worse than sandstone or common brick; it is well known that stone or brick walls are too permeable to allow plastering directly on the inside surface, and must be furred and lathed before plastering, to avoid dampness. This practice is generally followed with concrete blocks, but their use and popularity would be greatly increased if they were made sufficiently waterproof to allow plastering directly on the inside surface.
For this purpose it is not necessary that blocks should be perfectly waterproof, but only that the absorption of water shall be slow, so that it may penetrate only part way through the wall during a long continued rain. Walls made entirely watertight are, in fact, objectionable, owing to their tendency to "sweat" from condensation of moisture on the inside surface. For health and comfort, walls must be slightly porous, so that any moisture formed on the inside may be gradually absorbed and carried away.
Excessive water absorption may be avoided in the following ways:
1. Use of Properly Graded Materials. It has been shown by Feret and others that porosity and permeability are two different things; porosity is the total proportion of voids or open spaces in the mass, while permeability is the rate at which water, under a given pressure, will pass through it. Permeability depends on the size of the openings as well as on their total amount. In two masses of the same porosity or percentage of voids, one consisting of coarse and the other of fine particles, the permeability will be greater in the case of the coarse material. The least permeability, and also the least porosity, are, however, obtained by use of a suitable mixture of coarse and fine particles. Properly graded gravel or screenings, containing plenty of coarse fragments and also enough fine material to fill up the pores, will be found to give a much less permeable concrete than fine or coarse sand used alone.
2. Use of Rich Mixtures. All concretes are somewhat permeable by water under sufficient pressure. Mixtures rich in cement are of course much less permeable than poorer mixtures. If the amount of cement used is more than sufficient to fill the voids in the sand and gravel, a very dense concrete is obtained, into which the penetration of water is extremely slow. The permeability also decreases considerably with age, owing to the gradual crystallization of the cement in the pores, so that concrete which is at first quite absorbent may become practically impermeable after exposure to weather for a few weeks or months. There appears to be a very decided increase in permeability when the cement is reduced below the amount necessary to fill the voids. For example, a well-mixed sand and gravel weighing 123 pounds per cubic foot, and therefore containing 25 per cent voids, will give a fairly impermeable concrete in mixtures up to 1 to 4, but with less cement will be found quite absorbent. A gravel with only 20 per cent voids would give about equally good results with a 1 to 5 mixture; such gravel is, however, rarely met with in practice. On the other hand, the best sand, mixed fine and coarse, seldom contains less than 33 per cent voids, and concrete made from such material will prove permeable if poorer than 1 to 3.
3. Use of a Facing. Penetration of water may be effectively prevented by giving the blocks a facing of richer mixture than the body. For the sake of smooth appearance, facings are generally made of cement and fine sand, and it is often noticed that these do not harden well. It should be remembered that a 1 to 3 sand mixture is no stronger and little if any better in water absorption than a 1 to 5 mixture of well-graded sand and gravel. To secure good hardness and resistance to moisture a facing as rich as 1 to 2 should be used.
4. Use of an Impervious Partition. When blocks are made on a horizontal face machine, it is a simple matter, after the face is tamped and cores pushed into place, to throw into each opening a small amount of rich and rather wet mortar, spread this fairly evenly, and then go on tamping in the ordinary mixture until the mold is filled. A dense layer across each of the cross walls is thus obtained, which effectually prevents moisture from passing beyond it. A method of accomplishing the same result with vertical face machines, by inserting tapered wooden blocks in the middle of the cross walls, withdrawing these blocks after tamping, and filling the spaces with rich mortar, has been patented. In the two piece system the penetration of moisture through the wall is prevented by leaving an empty space between the web of the block and the inside face, or by filling this space with rich mortar.
5. Use of Waterproof Compounds. There are compounds on the market, of a fatty or waxy nature, which, when mixed with cement to the amount of
[698]
STONE
only 1 or 2 per Cent of its weight, increase its water resisting qualities in a remarkable degree. By thoroughly mixing 1 to 2 pounds of suitable compound with each sack of cement used, blocks which are practically waterproof may be made, at very small additional cost, from 1 to 4 or 1 to 5 mixtures. In purchasing waterproof compound, however, care should be taken to select such as has been proved to be permanent in its effect, and some of the materials used for this purpose lose their effect after a few days' exposure to weather, and are entirely worthless.
6. Application to Surface after Erecting. Various washes, to make concrete and stone impervious to water, have been used with some success. Among these the best known is the Sylvester wash of alum and soap solution. It is stated that this requires frequent renewal, and it is hardly likely to prove of any value in the concrete industry. The writer's experience has been that the most effective remedy, in case a concrete building proves damp, is to give the outside walls a very thin wash of cement suspended in water. One or two coats will be found sufficient. If too thick a coating is formed it will show hair cracks. The effect of the cement wash is to make the walls appear lighter in color, and if the coating is thin the appearance is in no way injured.
General Hints on Waterproof Qualities. To obtain good water-resisting properties the first precaution is to make the concrete sufficiently wet. Dry tamped backs, even from rich mixture, will always be porous and absorbent, while the same mixture in plastic condition will give blocks which are dense, strong, and watertight. The difference in this respect is shown by the following tests of small concrete blocks, made by the writer. The concrete used was made of 1 part cement and 5 parts mixed fine and coarse sand, by weight.
No. 1. With 8 per cent water, rather dryer than ordinary block concrete, tamped in mold.
No. 2. With 10 per cent % water, tamped in the mold, and the mold removed at once.
No. 3. With 25 per cent water, poured into a mold resting on a flat surface of dry sand; after 1 hour the surface was troweled smooth; mold not removed until set.
These blocks were allowed to harden a week in moist air, then dried. The weights, voids, and water absorption were as follows:
|
|
1 Damp Tamped |
2 Wet Tamped |
3 Poured |
|
Weight, per cubic foot, pounds |
122.2 |
123.9 |
110.0 |
|
Voids, calculated, per cent of |
25.9 |
24.9 |
33.3 |
|
Water required to fill voids, per cent of weight |
9.8
|
9.4
|
12.5
|
|
Water absorbed, after 2 hours, per cent of weight |
8.8 |
6.4 |
10.5 |
The rate at which these blocks absorbed water was then determined by drying them thoroughly, then placing them in a tray containing water | inch in depth, and weighing them at intervals.
|
|
1 Damp Tamped |
2 Wet Tamped |
3 Poured |
|
1/2hour 1 hour 2 hours 4 hours 24 hours 48 hours |
2.0 3.2 4.1 5.2 6.1 6.4 |
0.9 1.1 1.6 2.0 3.4 4.3 |
1.8 2.5 3.2 3.8 7.0 7.5 |
These figures show that concrete which is sufficiently wet to be thoroughly plastic absorbs water much more slowly than dryer concrete, and prove the importance of using as much water as possible in the damp-tamping process.
Cost. Concrete blocks can be sold and laid up at a good profit at 25 cents per cubic foot of wall. Common red brick costs (at this writing) generally about $12 per thousand, laid. At 24 to the cubic foot, a thousand brick are equal to 41.7 cubic foot of wall; or, $12, 29 cents per cubic foot. Brick walls with pressed brick facing cost from 40 cents to 50 cents per cubic foot, and dressed stone from $1 to $1.50 per foot.
The factory cost of concrete blocks varies according to the cost of materials. Let us assume cement to be $1.50 per barrel of 380 pounds, and sand and gravel 25 cents per ton. With a 1 to 4 mixture, 1 barrel cement will make 1,900 pounds of solid concrete, or at 130 pounds per cubic foot, 14.6 cubic feet. The cost of materials will then be:
Cement, 380 pounds $1.50
Sand and gravel, 1,500 pounds 0.19
Total $1.69
or 11.5 cents per cubic foot solid concrete. Now, blocks 9 inches high and 32 inches long make 2 square feet of face of wall, each. Blocks of this height
[699]
STONE
and length, 8 inches thick, make 1 1/3 cubic feet of wall; and blocks 12 inches thick make 2 cubic feet of wall. From these figures we may calculate the cost of materials for these blocks, with cores or openings equal to 1/3 or 1/2 the total volume, as follows:
Per cubic foot of block, 1/3 opening 7.7 cts.
Per cubic foot of block, 1/2 opening 5.8 cts.
Block 8 x 9 x 32 inches, 1/3 opening 10.3 cts.
Block 8 x 9 x 32 inches, 1/2 opening 7.7 cts.
Block 12 x 9 x 32 inches, 1/3 opening 15.4 cts.
Block 12 x 9 x 32 inches, 1/2 opening 11.6 cts.
If one-third of the cement is replaced by hydrate lime the quality of the blocks will be improved, and the cost of material reduced about 10 per cent. The cost of labor required in manufacturing, handling, and delivering blocks will vary with the locality and the size and equipment of factory. With hand mixing, 3 men at an average of $1.75 each will easily make 75 8-inch or 50 12-inch blocks, with 1/3 openings, per day. The labor cost for these sizes of blocks will therefore be 7 cents and 10 1/2 cents respectively. At a factory equipped with power concrete mixer and cars for transporting blocks, in which a number of machines are kept busy, the labor cost will be considerably less. An extensive industry located in a large city is, however, subject to many expenses which are avoided in a small country plant, such as high wages, management, office rent, advertising, etc., so that the total cost of production is likely to be about the same in both cases. A fair estimate of total factory cost is as follows:
Material Labor Total
8 x 32 inch, 1/3 space 10.3 7 17.3 cts.
8 x 32 inch, 1/2 space 7.7 6 13.7 cts.
12 x 32 inch, 1/3 space 15.4 10.5 25.6 cts.
12 x 32 inch, 1/2 space 11.6 9 20.6 cts.
With fair allowance for outside expenses and profit, 8-inch blocks may be sold at 30 cents and 12-inch at 40 cents each. For laying 12-inch blocks in the wall, contractors generally figure about 10 cents each. Adding 5 cents for teaming, the blocks will cost 55 cents each, erected, or 27 1/2 cents per cubic foot of wall. This is less than the cost of common brick, and the above figures show that this price could be shaded somewhat, if necessary, to meet competition. S. B. Newberry in a monograph issued by the American Association of Portland Cement Manufacturers.
Artificial Marbles.
I. The mass used by Beaumel consists of alum and heavy spar (barium sulphate) with addition of water and the requisite pigments. The following proportions have been found to be serviceable: Alum, 1,000 parts; heavy spar, 10 to 100 parts; water, 100 parts; the amount of heavy spar being governed by the degree of translucence desired. The alum is dissolved in water with the use of heat. As soon as the solution boils the heavy spar is mixed in, stirred with water and the pigment; this is then boiled down until the mixture has lost about 3 per cent of its weight, at which moment the mass exhibits a density of 34º Bé. at a temperature of 212º F. The mixture is allowed to cool with constant stirring until the substance is semi-liquid. The resultant mass is poured into a mold covered on the inside with several layers of collodion and the cast permitted to cool completely in the mold, whereupon it is taken out and dried entirely in an airy room. Subsequently the object may be polished, patinized, or finished in some other way.
II. Imitation Black Marble. A black marble of similar character to that exported from Belgium the latter product being simply prepared slate may be produced in the following manner: The slate suitable for the purpose is first smoothly polished with a sandstone, so that no visible impression is made on it with a chisel this being rough after which it is polished finely with artificial pumice stone, and lastly finished with extremely light natural pumice stone, the surface then presenting a soft, velvet-like appearance. After drying and thoroughly heating the finely polished surface is impregnated with a heated mixture of oil and fine lampblack. This is allowed to remain 12 hours; and, according to whether the slate used is more or less gray, the process is repeated until the gray appearance is lost. Polishing thoroughly with emery on a linen rag follows, and the finishing polish is done with tin ashes, to which is added some lampblack. A finish being made thus, wax dissolved in turpentine, with some lampblack, is spread on the polished plate and warmed again, which after a while is rubbed off vigorously with a
[700]
STOPPERS - STOVE POLISH
clean linen rag. Treated thus, the slate has the appearance of black marble.
STONE CEMENTS:
See Adhesives.
STONE CLEANING:
See Cleaning Preparations and Methods.
STONES FOR SHARPENING:
See Tool Setting and Whetstones.
STONES (PRECIOUS), IMITATION OF:
See Gems, Artificial.
STONEWARE:
See Ceramics.
STONEWARE CEMENTS:
See Adhesives and Lutes.
STOPPERS.
I. To make an anti-leak and lubricating mixture for plug cocks use 2 parts of tried suet and 1 part of beeswax melted together; stir thoroughly, strain, and cool.
II. A mixture for making glass stoppers tight is made by melting together equal parts of glycerine and paraffine.
To Loosen a Glass Stopper.
I. Make a mixture of
Alcohol 2 drachms
Glycerine 1 drachm
Sodium chloride 1 drachm
Let a portion of this stand in the space above the stopper for a few hours, when a slight tap will loosen the stopper.
II. A circular adjustable clamp, to which is attached a strip of asbestos in which coils of platinum wire are imbedded, is obtained. By placing this on the neck of the bottle, and passing a current of electricity through the coils of wire, sufficient heat will be generated to expand the neck and liberate the stopper. Heat may also be generated by passing a yard of cord once around the bottle neck and, by taking one end of the cord in each hand, drawing it rapidly back and forth. Care should be taken that the contents of the bottle are not spilled on the hand or thrown into the face when the stopper does come out or when the bottle breaks.
STOPPER LUBRICANTS:
See Lubricants.
STOVE POLISH:
See also Polishes.
The following formula gives a liquid stove blacking:
Graphite, in fine powder 1 pound
Lampblack 1 ounce
Rosin 4 ounces
Turpentine 1 gallon
The mixture must be well shaken when used, and must not be applied when there is a fire or light near on account of the inflammability of the vapor.
This form may be esteemed a convenience by some, but the rosin and turpentine will, of course, give rise to some disagreeable odor on first heating the stove, after the liquid is applied.
Graphite is the foundation ingredient in many stove polishes; lampblack, which is sometimes added, as in the foregoing formula, deepens the color, but the latter form of carbon is of course much more readily burned off than the former. Graphite may be applied by merely mixing with water, and then no odor follows the heating of the iron. The coating must be well rubbed with a brush to obtain a good luster.
The solid cakes of stove polish found in the market are made by subjecting the powdered graphite, mixed with spirit of turpentine, to great pressure. They have to be reduced to powder and mixed with "water before being applied.
Any of them must be well rubbed with a brush after application to give a handsome finish.
STOVE CEMENT:
See Cement.
STOVE CLEANERS:
See Cleaning Compounds.
STOVE LACQUER:
See Lacquers.
STOVE VARNISHES:
See Varnishes.
STRAMONIUM, ANTIDOTE FOR:
See Atropine.
STRAP LUBRICANT:
See Lubricant.
STRAW FIREPROOFING:
See Fireproofing.
STRAWBERRIES, FROZEN:
See Ice Creams.
STRAWBERRY JUICE:
See Essences and Extracts.
STRAW HAT CLEANERS:
See Cleaning Preparations and Methods.
STRAW HAT DYES:
See Hats.