 The
Science Notebook
The
Science NotebookStatic Electricity
 The
Science Notebook
The
Science NotebookHome Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!
Electricity and magnetism are vital to our modern
world. Without them, we would still be warming
ourselves with open fires and would not enjoy the many
electrical and electronic devices we take for granted every
day. Imagine a world without television, electric
lights, computers, or air conditioning. Yet without an
understanding of electricity and magnetism, none of these
inventions would have ever been possible. On the
Electricity and Magnetism pages, we will follow in the
footsteps of many scientists as we explore these two
fascinating subjects.
The first type of electricity to be discovered was
static electricity. The discovery of static
electricity was mankind’s first step toward learning about
electricity.
Many years ago, it was found
that certain objects were given an electric charge simply by
rubbing them with certain other objects. These
objects, when charged, would then attract other
objects. This charge came to be known as “static
electricity”. This first series of experiments
illustrate some of the properties of static
electricity. They are best done on a cool dry day, but
in summertime, they will often work in an air-conditioned
room as well.

Ever have a bad hair day?
Try this, and the day won’t be a total loss!
Materials Needed:
Plastic comb; paper.
Procedure:
Tear off several bits of paper about the size of a pea or
smaller and place them on a table or counter. Bring the comb
near to the paper bits. What happens?
Next, run the comb through your hair several times and again
bring the comb near the bits of paper. What happens now?
What Happened: The
first time you brought the comb near the paper bits, nothing
happened, but when you combed your hair, you gave the comb a
charge of static electricity Then, when you brought the comb
near the paper bits, they were attracted to the comb because
of this charge. We’ll see what caused the attraction a
little later.
You may have noticed a crackling sound when you
pulled off a sweater on a cold day. This is another
example of static electricity. It works best on a dry
day in winter.
Materials Needed:
Sweater; dark room or closet; mirror.
Procedure:
Pull off your sweater in a dark room or closet while watching
in the mirror.
What Happened: If you
heard crackling, and if the room or closet was dark enough,
you should have seen tiny sparks! As you pulled off your
sweater, very tiny particles called “electrons” moved in
streams between your sweater and your body. You may have
even felt your hair stand on end. Each of these streams
of electrons jumping between your body and the sweater were
actually miniature sparks of lightening.

You’ve probably done this experiment many times
before without ever intending to!
Materials Needed: A
carpeted room on a dry day; a doorknob; yourself.
Procedure: Walk
across the carpeted floor. Touch a metal doorknob.
What do you see, hear and feel?
What Happened: You
probably felt a nice little shock! As you walked across the
carpet, you picked up loose electrons. When you brought
your hand to the door, those extra electrons “jumped” over to
the metal doorknob and a spark was created.
Going Further: Try
this in a dark room, and you should have no trouble seeing the
spark. Also, try touching another person after walking
across the room, but be nice... let them know what you’re
going to do first!
Materials
Needed: Balloon; smooth wall.
Procedure:
Blow up the balloon and tie it off. Now rub it several
times across your hair and then press it against the
wall. What happens?
What Happened: The
balloon received a charge of static electricity as it rubbed
against your hair. This electric charge caused it to be
attracted to the wall.
Going Further: Leave
the balloon in place for a while. How long will it stay
there if left undisturbed?
So far, we have seen static electric charges
generated on your body. In this experiment, we’ll look
at one example of static electricity generated outside of
your body.
Materials Needed: Two
books; glass from a picture frame; paper; piece of flannel or
silk cloth.
Procedure: Clean the
glass with a good glass cleaner and allow it to dry
thoroughly. Place the two books down side by side with a
gap between them just a little less than the width of the
glass. Tear several bits of paper about the size of a
pea and place them between the books. Place the glass on
the books so that it is centered over the paper bits.
Now rub the cloth across the top of the glass. What
happens?
What Happened: As you
rubbed the glass, it was charged by the cloth. The
charged glass then attracted the paper bits, but as each of
the paper bits touched the glass, they fell back. This is
because the paper had a weak charge that was opposite of the
glass. Since opposite charges attract, the paper was
drawn to the glass. However, when the paper touched the
glass, its charge was neutralized. The paper was no
longer was attracted to the glass, and so fell away.
Investigating
the
Effect
of Static Electricity on Water

Materials
Needed: Comb; faucet.
Procedure:
Just barely open a water faucet so that you get a very fine
stream of water flowing. Then, run the
comb through your hair several times. Slowly bring the
comb near the stream of water. What do you see?
What Happened: From
previous experiments, you already know that the comb is
charged with static electricity. When you bring the comb
near the stream of water, you see it bend toward the
comb. There is a slight charge on each of the water
molecules that draws them toward the charged comb.

Materials
Needed: Meter or yard stick, or dowel, or other
long stick; two chairs; two balloons; string; piece of glass
from a picture frame; flannel or silk cloth.
Procedure:
Blow up the two balloons and tie each off with a length of
string. Place the stick across the two chairs and suspend the
two balloons so that they hang freely about two inches apart.
Rub each balloon across your head several times, and then
allow the balloons to hang freely again. What
happens? Next, rub the piece of glass with the cloth,
and then bring the piece of glass between the two
balloons. What happens now?
What Happened: Rubbing
the two balloons on your head gave each a negative charge. The
two balloons then had like charges, and this caused them to
repel each other. Like
charges repel.
When you rubbed the glass with the flannel or silk, you gave
it a positive charge. When you put the glass between the
balloons, the negatively charged balloons were attracted to
the positively charged glass because unlike charges attract.
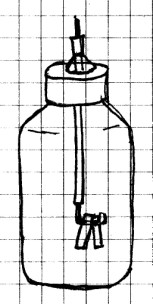
An electroscope is a device that will detect very
small charges of static electricity.

Materials Needed: ½
liter (16-20 ounce) plastic or glass soft drink bottle with
plastic screw cap; piece of insulated heavy gauge copper wire;
aluminum foil; modeling clay; plastic comb.
Procedure: Wash the
bottle and remove the label. Allow the bottle to dry
thoroughly. Using a sharp nail, punch a hole in the
center of the plastic cap. This hole should be just a
little larger than the wire you are using.
Cut a piece of wire just a little shorter than the
bottle. Remove about 2 cm (½ inch) of insulation from
one end and about 4 cm (1 in) from the other end. Using a pair
of pliers, bend the end with 4 cm (1 in) stripped off into a
squared “J” shape as shown below.
From underneath the cap, stick the straight portion of the
wire through the hole in the cap so that about 4 cm (1 in) or
so of insulated wire is above the cap. Place a small amount of
modeling clay around the wire on top of the cap to hold it
firmly in place.
Next, cut a piece of aluminum foil about 15 cm (6 in) long and
1 cm (1/4 in) wide. (You should use thin foil rather
than the thicker “heavy duty” type.) Fold this strip in
half and cut it in two at the fold. Punch a hole near
one end of each piece of foil. Hang each piece of foil
over the “J” on the wire. Make sure that the two halves
of the foil are close together but that they do not quite
touch. Insert the foil and wire into the bottle and
screw on the cap.
 Now
comb
you hair several times with the comb. Bring the comb
near the bare wire at the top. Watch the foil as you
do. What happens?
Now
comb
you hair several times with the comb. Bring the comb
near the bare wire at the top. Watch the foil as you
do. What happens?
What To Look For: The
foil halves should repel each other.
What Happened: The
comb was charged with a negative charge. This negative
charge was created on the comb as it picked up extra electrons
from your hair. When the comb was brought close to the
copper wire, some of the electrons moved from the comb to the
wire and from there down to the foil. When the electrons
reached the foil, they spread out over both pieces of the
foil. Since both sides of the foil then had a like
charge (negative), the sides repelled each other.
Going Further: Try
charging a drinking glass with a flannel or silk cloth.
What happens if you bring the glass near to the wire while the
leaves of foil are spread out from the negative charge?
Why?
There's much more to learn about electricity.
For more interesting experiments, visit the Current
Electricity and Simple Circuits page.