The
Science Notebook
Gilbert Chemistry - Part 2
The
Science Notebook
Gilbert Chemistry - Part 2
NOTE: This book was published in 1936 as a manual
to accompany several Gilbert Chemistry sets of the time.
While some of the experiments and activities here
may be safely done as written, a number of them use chemicals
and methods no longer considered safe. In
addition, much of the information contained in this book about
chemistry and other subjects is outdated and inaccurate.
Therefore, this book is probably best appreciated for
its historical value rather than as a source for current
information and good experiments. If you try
anything here, please understand that you do so at
your own risk. See our Terms of Use.
Pages 21-40
[21]
EXPERIMENT Page
597. Shoddy - 172
Source of Cotton - 172
598. Cotton industries - 172
599. Cotton plant - 172
Source of Silk - 173
600. Cocoons - 173
601. Degumming - 173
Examination of Fabrics with the
Microscope - 173
602. Wool fiber - 173
603. Cotton fiber - 173
Chemical Identification of Textile Fibers
- 173
604. Identification of wool - 173
605. Solubility of wool in alkali - 173
606. Identification of cotton - 173
607. Action of alkali on cotton - 174
608. Action of alkali on silk - 174
609. Action of alkali and copper sulphate on cotton
- 174
The Chemistry of the Body - 174
610. How to test for sugar in urine - 176
611. How to test for albumen in urine - 177
612. How to test for proteins in urine - 177
613. Testing urine for acidity - 177
614. Testing urine for ammonia - 177
615. Testing urine for phosphate - 178
616. How to test for acid mouth - 178
The Chemistry of Plants-Agriculture
- 178
The Chemistry of Fertilizers-Farming
- 178
617. Nitrogen forming bacteria - 180
618. To show the effect of carbon dioxide on plant life
- 180
A SIMPLE PHOTOCHEMICAL EXPERIMENT TO DETERMINE
THE ACTIVITY OF THE
ENERGY OF THE SUN'S RAYS
619.
"Cold Light," or Light by Chemical Action
- 182
620. A demonstration of "Chemiluminescence - 182
Luminol - 182
PART IV
ELECTRO-CHEMISTRY
The Dry Cell and How It Is Made- 184
How the Dry Cell Works - 185
The Wet Cell - 186
The Storage Battery - 187
621. How to test a battery of dry cells - 187
622, How to determine the positive or negative wire
- 188
623. Another way to tell the positive and negative wires
- 188
624. How to show the direction of a current - 189
Electroplating - 189
625. How to copper-plate - 190 |
[22]
EXPERIMENT
Page
626. How to nickel-plate - 191
Electrotyping - 191
627. How to reproduce a medal - 192
628. How to make a bronze statue from a plaster cast
- 192
Etching by Means of Electricity
- 193
629. How to etch on copper - 193
630. How to etch on steel - 193
631. Copper-plating by immersion - 194
632. Tin-plating by contact - 194
633. Nickel-plating by contact - 194.
634. Formation of a current by contact of copper with zinc
- 194
635. Formation of a current by contact of silver with zinc
- 194
Electrolysis - 194
636. The electrolysis of sodium chloride - 195
637. The lemon electric cell - 195
638. How to clean silverware electrolytically - 195
639. How to galvanize iron with zinc - 196
640. How to galvanize iron with nickel - 196 |
[23]
INTRODUCTION
The object in writing this book is
to present the simple fundamental concepts of the Science of
Chemistry in a form which will appeal to boys, and arouse in them
a desire to acquire an understanding and appreciation of some of
the fundamental laws of nature. The subject matter has
necessarily been arranged and presented in a style to stimulate
the boy's interest and curiosity without creating the feeling, on
the part of the boy, that he is undertaking a laborious problem
which will not give him pleasure. It is earnestly hoped that
the subject matter will provide the opportunity for any boy to
have a lot of fun in doing the many experiments, and by so doing,
obtain an elementary knowledge of the principles upon which the
Science of Chemistry is based.
[24]
ORIGIN
OF THE WORD "CHEMISTRY"
The first literary work in which the word - "Chemistry" - is found
was written by Plutarch, a Roman historian who lived from 46-120
A.D. In a treatise entitled - “Isis and Osiris"-that philosopher
mentions that "Egypt" in the dialect of the country, was called the
same name as the black of the eye, "Chemia," and from this be infers
that the word meant "Black" in the Egyptian language. Some science
historians believe that our word "Chemistry" means "The Egyptian
Art." Others think that the word was coined to mean "The black art."
Still others think that the word meant "The dark or hidden
art." Another school of thinkers believes that the word
has no connection with Egypt at all, but that it comes from the
Hebrew word-"Chaman," meaning mystery. Another possible derivation,
according to some historians, is from the Arabic word
"Chema"-meaning to hide, hence "the Hidden Science." ln fact, a book
of secrets was written in the time of the ancient Arabians called
"Kemi." Probably no one will ever know definitely which one of these
possible derivations is the correct one.
ORIGIN OF
CHEMISTRY AS A SCIENCE
According to some historians, the origin of chemistry as a science
dates back to the time of Tubal Cain, the father of workers in
metal. Credit is also given to Hermas, the Egyptian god of art and
sciences. His son is said to have colonized Egypt, which was
foremost in the knowledge of chemistry in those ancient days for
they had developed the arts of making glass, pottery, colors,
embalming fluids and other practical products to a high degree, and
the early Egyptians can really be said, therefore, to have had an
advanced knowledge of applied chemistry. Then Paracelsus, the Greek
physician, carried the study along and discovered the influence of
chemistry upon medicine in the treatment of human ills, and it was
through him that the action of several inorganic salts upon the
human systems became known. Following this period a long time
elapsed, hundreds of years, during which time contributions were
spasmodically made by unknown workers in science, but which really
had little influence upon the development of modern chemistry.
Chemistry, as we know it today, is one of the newest of our
sciences, and yet it is one which offers the greatest opportunities
of advancement, research and fame for those today who are interested
in the fuller things of life. Centuries ago there was no such thing
as chemistry. Chemistry was preceded by alchemy. Alchemists were
superstitious men and were very often dishonest men. He was a groper
for mysteries, and if it had not been for this interest in the
mysteries of energy and matter, modern science would never have been
born. We can now visualize the old alchemist working over his pots
and retorts in crude laboratories and in dark caves. Shrouded in
mysticism, and his activities kept secret, his imagination fired
with zeal and exercising patience, and with the purpose of a
religious fanatic, he sought to make or find the philosopher's
stone.
It was not until the early part of the eighteenth century that the
scientist of the central European countries and the English Empire
began to contribute fundamental knowledge which laid the foundation
and paved the way for the development of this wonderful
science. The Frenchman, Lavoisier (1743-94) may really
be credited with being the father of modern chemistry.
There is hardly a science today that has greater economic influence,
or holds more fascinating interest to scientists throughout the
world than chemistry. If we are to
GILBERT
CHEMISTRY 25
unravel the secrets of our wonderful world and life, there is no
science that will enable us to understand and correctly interpret
these hidden things of nature that most of us think are magical and
mysterious, like a knowledge of chemistry.
No large and progressive manufacturing industry can cope with its
competitors today without a trained chemist to advise and assist in
its development and the analysis of the raw materials which it buys.
The present-day physician without a knowledge of chemistry would be
incompetent and unable to maintain an acceptable professional
standing as a practitioner of medicine.
The great problems involved in the manufacture of synthetic drugs,
dyes, perfumes, essential oils, of soil fertilization, and of the
many substituted and artificial productions influencing modern
civilization are every-day problems of chemistry. The regulation of
our food supply calls for the services o thousands of experienced
technicians who are employed as chemists by industry, municipalities
and both our State and National governments. If we would have
our country today improve its standards of living and at the same
time accommodate itself to an increasing population, we must
hereafter maintain on an even more liberal scale than ever before
great laboratories of science devoted to the study of
chemistry. The men and women working in these laboratories are
among our priceless possessions. There is no sum that the world
could not afford to pay these men who have originality of mind and
devotion and industry to carry forward in scientific advancement
until its influence spreads to the comfort of every home. It was
former President Coolidge who wrote as follows: "Wherever we look,
the work of the chemist has raised the level of our civilization,
and has increased the productive capacity of our nation.
Probably most boys are interested in science because they just
naturally think they will like science. This is a perfectly good and
sufficient reason in itself. At the same time, a boy of intelligence
who becomes interested in science would like to be reassured, no
doubt, that science offers a really important field for service in
the interests of human welfare. From the far-sighted point of
view the public is better off today than it was before science was
developed, and so it always will be. Every boy and girl should
be impressed with this fact and made to realize that science creates
jobs, and that its application makes life more comfortable and more
interesting.
In order to illustrate this point of view, emphasis has been laid on
experiments in this little manual which show the relationship
between chemistry and its application to our chemical industries and
to everyday life. There was a time when chemistry was regarded as
being related to witchery and sorcery. Chemicals were formerly
looked upon as deadly poisons and chemical reactions were associated
with explosions. The men who practiced the science of chemistry had
to do so in secret because they were regarded by people with
superstition and as related to the devil.
Today conditions are entirely different. There is now no need for
secrecy. A chemist is looked upon today as a professional man to be
treated with respect, and there is a growing desire to know more
about this science. To satisfy to some degree the youthful thirst
for chemical knowledge, and to afford the pleasure to boys to be
derived from the intelligent performance of simple experiments, is
one of the aims of this manual. The second aim is to develop
the power of scientific reasoning and to give to the the boy an
elementary knowledge of the fundamental principles upon which modern
chemistry is based.
The experiments in this manual must be carried out with accuracy in
order to obtain satisfactory results. Remember that nature is
exacting in her method of operation, and it is the problem of the
scientist to seek the truth and operate according to the "rules of
the game," so to speak, by careful experimentation. The author would
therefore urge that you think out for yourself, when you are
performing experiments, first as to what
26
GILBERT CHEMISTRY
you are doing the experiment for, second, weigh carefully the
results obtained, and third, draw some conclusions as to what the
results really mean to you. lt is by so doing that you will develop
your imagination, and an investigative mind. The performance of your
experiments will prove a pleasure to you, an at the same time you
will contribute to your knowledge and also advance and develop the
science of chemistry.
WARNING!
Gilbert Chemistry Sets are not intended for children who cannot read
and understand the accompanying Instruction Books.
Gilbert Chemistry Sets do not contain dangerous poisons and the
chemicals mentioned in this manual are not embraced under the term
"poisons." They are perfectly safe to use if handled carefully and
intelligently. They are not intended to be taken by mouth or
swallowed, and no intelligent person would be expected to use them
for such purposes. It is necessary, however, to emphasize the fact
that carelessness on the part of the experimenter can always lead to
trouble. The author suggests, therefore, that all experimentation be
carried out cautiously and according to the directions, especially
when manipulations like heating is involved, or when gases are
evolved in the reactions.
Before performing any experiments outlined in the manual, the
following instructions should be read carefully and observed.
Before performing experiments, be sure to spread a thick layer of
newspapers or other protective material over the table, so that hot
liquids, candle grease, etc., will not injure the table.
Always read an experiment entirely through before starting to
perform it. By following this rule many mistakes may be avoided.
Never point the open end of a test tube, while heating, at yourself
or anyone nearby, as it may suddenly boil over, causing burns or
iniuring clothing. For the same reason never smell at the open end
of a test tube while heating, or put your face near it.
GILBERT
CHEMISTRY 27
DEFINITIONS
OF SOME COMMON CHEMICAL TERMS
ACIDS
Chemical compounds which have a sour taste. They turn blue litmus
red. They unite with bases to form salts. They all contain hydrogen,
which is a gas.
ATOM
The smallest particle of an element which enters into chemical
combination. Atoms are extraordinarily small. We can never hope to
see one, even with powerful magnifying glasses.
ATOMIC WEIGHT
Relative weight of an atom compared with an atom of hydrogen as a
standard. Since hydrogen is the lightest known element, the weight
of its atom is taken as 1. When we say that the atomic weight
of oxygen is 16 we mean that the atoms of oxygen are 16 times
heavier than those of hydrogen.
BASE
A base is water in which half of the hydrogen has been replaced by a
metal. For example, water is H/OH. A base, sodium hydroxide is Na/
OH. Bases are also known as alkalies. They combine with
acids to form a salt of the metal and water.
CHEMISTRY
The science of chemistry has for its object the accurate
investigation of all changes in the identity of substances and the
laws, causes and effects of such changes.
CHEMICAL CHANGE
A change which destroys the identity of the substance or substances
acted upon.
CHEMICAL COMPOUND
A union of two or more substances in definite quantities, combined
so as to form a new and distinct substance which is unlike either of
the substances which formed it.
CHEMICAL EQUATION
When chemical substances react upon or unite with one another,
definite transformations take place which can be expressed in the
form of a chemical equation. Thus we may express the action of
hydrochloric acid on calcium carbonate to form calcium chloride.
water and carbon dioxide as follows:
2HCl + CaCO3 = CaCl2
+ H2O +CO2
An equation is an abbreviated form of what takes place in a chemical
reaction.
CHEMICAL AFFINITY
Property which elements have for uniting with one another.
DECOLORIZE
To bleach or whiten - to remove the color from a liquid or solid.
DECOMPOSE
The process by which a compound breaks up into simpler parts -
usually through the action of heat.
DEODORIZE
To remove an odor or smell - especially the odor which results from
impurities.
DISSOCIATE
The process by which a compound breaks up into ions when dissolved
in water.
ELECTROLYSIS
The decomposition or breaking up of a chemical compound by means of
an electric current.
28
GILBERT CHEMISTRY
ELEMENT
A substance which cannot be separated into simpler parts.
EVAPORATE
To change a liquid or solid into a vapor or gas. This is usually
done with heat. Minerals, salts or ash often remain behind.
Many liquids will evaporate on simple exposure to the air.
IMMERSE
To dip or plunge into anything that surrounds or covers - especially
a liquid.
ION
An atom or group of atoms which carries a certain amount of
electricity.
LAW OF CONSERVATION OF MATTER
Matter can neither be created nor destroyed. For example, if we burn
a piece of coal, the weight of ashes and gases formed after burning
is exactly equal to the weight of the coal before burning. This is
true with every chemical reaction that takes place.
LAW OF DEFINITE PROPORTIONS
Atoms unite with one another in definite though frequently in two or
more different proportions. For example, carbon, sulphur and arsenic
form two distinct oxides, CO and CO2, SO3 and
SO3 and As2O3 and As2O5.
MIXTURE
A mass of two or more ingredients, the particles of which are
separate, independent and uncompounded with each other, no matter
how thoroughly and finely they are mixed. There is no chemical union
as there is in a compound.
MOLECULE
The smallest particle of a chemical compound which is capable of
existence.
PHYSICAL CHANGE
A change which does not affect the identity of the substance or
substances acted upon.
PRECIPITATE
An insoluble substance separated from a solution by the action of
some substance which is added to the solution. The precipitate may
fall to the bottom (hence the name which means to throw down) or it
may float in the liquid.
SALTS
Compounds formed by the combination of acids and bases, and
resulting in the replacement of part or all of the hydrogen atoms of
the acid by metals.
SOLUTION
The art or process by which a body: whether solid, liquid or
gaseous, is absorbed in a liquid and diffused or spread throughout
the liquid. The liquid is called the solvent.
SYMBOL
For convenience, elements are designated by symbols. Each symbol
stands for one atom of an element; as S for sulphur, Pb for lead
(Latin plumbum). NaCl is the chemical formula of and represents a
molecule of sodium chloride or common table salt.
VALENCE
The combining power of an element. Chlorine is univalent and oxygen
is bivalent because they unite with hydrogen to form the molecules,
HCl (hydrochloric acid) and H2O (water),
respectively.
GILBERT
CHEMISTRY 29
CHEMISTRY
CLASSIFICATION
Inorganic Chemistry
Metals, minerals, etc., which are found in nature occurring in the
crust of the earth are classified as inorganic materials. They are
not combustible in the sense that they can be burned like carbon to
gaseous products. They represent a classification which was
originally spoken of as mineral substances and are distinguished
from those products or substances which originate directly or
indirectly from living organisms. Iron, copper, glass and the
ore pyrite, for example, are typical inorganic substances, and all
materials of this nature are treated under a specific classification
which we designate as inorganic chemistry
Organic
Chemistry
It was the French chemist, Lavoisier (1743-94) who showed that in
spite of their great numbers, nearly all vegetable products
occurring in nature are composed of three elements - carbon,
hydrogen, and oxygen - whereas animal substances, which also consist
for the most part, of these same three elements, contain nitrogen,
and in some cases, phosphorus, sulphur and iodine. All such products
were shown to be not only peculiar in their composition, but were
also combustible. This discovery of Lavoisier and later workers led
to the belief that all animal and vegetable substances in nature
were produced under the influence of a vital force, and that their formation in nature
was regulated by laws which were different from those which governed
the formation of mineral substances. For this reason,
therefore, compounds obtained from animals and plants, either
directly or indirectly, were called organic compounds and a study of
the products of this type was classified under the designation
"Organic Chemistry." This distinction between organic
chemistry and inorganic chemistry was generally accepted until the
year 1828, when the German chemist, Wohler, succeeded in preparing
urea (an excretion product of animal organisms) by heating the
inorganic salt, ammonium cyanate, a substance which might be
considered to be inorganic, or mineral. This classic synthesis
showed that the influence of a living organism was not necessary for
the production of an organic substance - urea. As the science of
chemistry was developed, it was soon found that a great many other
so-called organic substances could be prepared tn the laboratory by
artificial methods and from materials of inorganic origin, and
ultimately it came to be generally acknowledged that many of the
process which occur in animals and plants could very probably be
carried out in the laboratory, and that the formation of an organic
compound is probably not dependent at all on the help of any vital
force than is that of an organic compound. Today this difference
between the two classes of compounds has been recognized as an
imaginary one, and the terms "organic chemistry" and "inorganic
chemistry " have to a large degree, lost their original meanings.
They do, however, serve to sub-divide the fields of chemistry into
two groups which are characterized by their own special technique,
and whose exploitation has led to products which have satisfied many
human needs and produced a basis for important and basic chemical
industries. The compounds of carbon compounds also are all related
to one another and they differ widely in their general behavior from
those of all other elements. Carbon compounds, therefore, form, in
fact, a very distinct group of compounds, and it is therefore
convenient to class them separately and to distinguish them by the
term "organic." Organic chemistry, therefore, is, according to
modern interpretation, the chemistry of the carhon compounds.
30
GILBERT CHEMISTRY
METRIC SYSTEM
TABLES
Length
1 kilometer (km) = 1000 meters
1 hectometer (hm) = 100 meters
1 dekameter (dkm) = 10 meters
1 decimeter (dm) = 0.1 meter
1 centimeter (cm) = 0.01 meter
1 millimeter (mm) = 0.001 meter
Weight
1 metric ton(t) = 1000 kilograms
1 kilogram = 1000 grams
1 hectogram (hg) = 100 grams
1 dekagram (dkg) = 10 grams
l decigram (dg) = 0.1 gram (g)
1 centigram (cg) = 0.01 gram
1 milligram (mg) = 0.001 gram
Area
1 square kilometer (sq. km) = 1,000,000 square meters
1 square hectometer (sq. hm) or 1 hectare (ha) = 10,000
square meters
1 square dekameter (sq. dkm) or 1 are (a) = 100 square
meters
1 centare (ca) = 1 square meter
1 square decimeter (sq. dm) = 0.01 square meter
1 square centimeter (sq. cm) = 0.0001 square meter
1 square millimeter (sq. mm) = 0.000001 square meter or 0.01
square centimeter
Volume
1 cubic kilometer (cu. kl.) = 1,000,000,000 cubic meters
1 cubic hectometer (cu. hm) = 1,000,000 cubic meters
1 cubic dekameter (cu. dkm) = 1,000 cubic meters
1 cubic meter (cu. m) = 1 stere (s)
1 cubic decimeter (cu. dm) = 0.001 cubic meter or 1 1iter
1 cubic centimeter (cu. cm) or (cc) = 0.000001 cubic meter
or 1 milliter (ml)
1 cubic millimeter (cu. mm) = 0.00000001 cubic meter or
0.001 cubic centimeter
CONVERSION TABLES
Weight
10 grams = about one-third ounce
50 grams = about 2 ounces
250 grams = about one-half pound
Capacity
50 cubic centimeters (cc) = about 1 fluid ounce
60 cubic centimeters (cc) = about 2 fluid ounces
125 cubic centimeters (cc) = about 4 fluid ounces
250 cubic centimeters (cc) = about 8 fluid ounces
360 cubic centimeters (cc) = about 12 fluid ounces
500 cubic centimeters (cc) = about 16 fluid ounces
1000 cubic centimeters (cc) = about 32 Buid ounces
|
GILBERT
CHEMISTRY 31
Length
1 millimeter (mm) = about one twenty-fifth of an inch
1 centimeter (cm) = 10 millimeters = about two-fifths of an
inch
1 inch = about 2 1/2 centimeters
METRIC CONVERSION TABLE
millimeters / 25.4 = inches
centimeters / 2.54 = inches
meters x 39.37 = inches
meters x 1.094 = yards
square centimeters x .155 = square inches
square meters x 10.764 = square feet
square kilometers x 247.1 = acres
cubic centimeters / 16.383 = cubic inches
cubic centimeters / 3,69 = fluid drams
cubic centimeters / 29.57 fluid ounces
cubic meters x 35.315 = cubic feet
cubic meters x 1.308 = cubic yards
cubic meters x 264.2 = gallons (321 cubic inches)
liters x 61.022 = cubic inches
liters x 33.84 = fluid ounces
grams / 981 = dynes
grams (water) / 29.57 = fluid ounces
grams / 28.35 = ounces avoirdupois
grams per cubic centimeter / 27.7 = pounds per cubic inches
joule x .7373 = foot pounds
kilograms x 2.2046 = pounds
U.S. WEIGHTS AND MEASURES
Apothecaries' Weight
20 grains = 1 scruple
60 grains = 3 scruples = 1 drachm
480 grains = 24 scruples = 8 drachm = 1 oz.
5760 grains = 288 scruples = 96 drachm = 12 oz. = 1 lb.
Avoirdupois Weight
27.343 grains = 1 drachm
437.5 grains = 16 drachm = 1 oz.
7000 grains = 256 drachm = 16 oz. = 1 lb.
Troy Weight
24 grains = 1 dwt.
480 grains = 20 dwt. = 1 oz.
5760 grains = 240 dwt. = 12 oz. = 1 lb.
Imperial Fluid Measure
16 fluid drams = 1 fluid oz.
128 fluid drachms = 16 fluid oz. = 1 pint
1024 fluid drachms = 128 fluid oz. = 9 pints = 1 gallon
1 gallon = 58328.886 grains of water at 16.7 dg. C. = 62.06
dg. F.
1 fluid oz. = 455.694 grains of water at 16.7 dg. C. = 62.06
dg. F.
1 gallon = 231.000 cubic inches.
1 fluid oz. = 1.8047 cubic inches
1 cubic foot of water = 1000 oz. Avoir,
|
[32]
PART I
The Chemists’ Laboratory
and
How the Chemist Uses His
Equipment
The chemist‘s work-room or laboratory has several special
requirements if it is to be fully satisfactory. A room
somewhat isolated to avoid interruption is desirable, especially if
small children are around to stick inquisitive fingers into things.
Good ventilat1on is necessary, and at least enough heat at all times
to keep water solutions from freezing. While a capable chemist
seldom spills anything, and, in spite of popular opinion, almost
never has an explosion, it is better to have the laboratory plainly
and simply furnished so that an accidental splash will do no damage.
A plain wooden floor is better than a carpet, and concrete or
linoleum are still better. The work table may be of plain lumber,
with the top waxed frequently to protect it. A sink and a supply of
running water are quite essential, but if he lacks these the
ingenious boy chemist will find a way to provide himself with
running water from a pail fitted with a siphon and hose. And you
never will get too many shelves, cabinets and drawers for storage.
Now in picturing to you this ideal laboratory, we realize that few
boys can have all this at once. In fact, your Gilbert Chemistry Set
has been designed to be as far as possible a complete laboratory in
itself. But we feel sure you will enjoy it more if you can at
least select for it a secluded corner in den or kitchen, or even in
the woodshed, cellar, or attic, where your apparatus mag be left set
up undisturbed and where there will be room to expand as you build
or buy new equipment and supplies.
THE
EQUIPMENT AND ITS USE
Good technique can only be acquired by careful self-training. Learn
what use each piece of apparatus is intended for, and the best way
to handle or use it. Begin at the start by having a place to keep
each and every piece, and keep it clean and in its place. Be
extremely careful not to contaminate your chemical supplies by
getting even traces of one into the bottle with another. And watch
to keep your chemicals replaced as soon as the supply runs low.
While you have not been furnished with dangerous and poisonous
chemicals, nevertheless they are not intended to be taken into the
mouth, and you should begin now to train yourself, not only never to
taste anything in the laboratory, but to use caution in
smelling.
THE
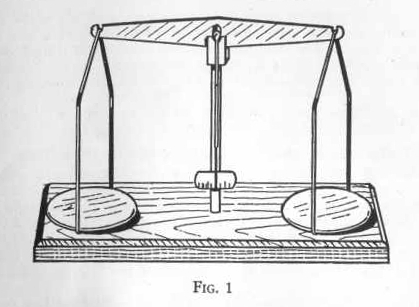
BALANCE
The balance is one of the most prized possessions of the chemist and
for very accurate weighing he may have a balance costing several
hundred dollars and sensitive to one ten-thousandth of a gram or
less.
Supplied with the balance are several weights weighing 1/2 gram
each. Since all chemists use the metric system to express
weights and measures you should familiarize yourself with the
simplest units. The gram is the unit of weight, and id taken as the
weight of 1 cubic centimeter of water at a temperature of
4° centigrade.
GILBERT
CHEMISTRY 33
1000 grams = 1 kilogram = 2.2
pounds (approximately)
1 /1000 gram = 1 milligram
These are the units of weight you will use most commonly. You may
also find it convenient to know that
1 pound = 454 grams (approximately)
1 ounce = 28 1/2 grams (approximately)
Abbreviations:
Kilogram - kg.
Gram - gm.
Milligram - mg.
Pound - lb.
Ounce - oz.
EXPERIMENT 1 - To weigh solids on
your balance
First protect the balance by covering both pans with squares of
paper of the same size. Be sure the beam is swinging freely without
friction and observe the center point of the pointer on the scale.
It should swing an equal distance each side of center of the scale
and if it does not you should look to see that the papers are equal
in weight.
Now place the required weights on the paper on the right pan, and
carefully pile the substance to be weighed on the left pan until the
pointer again swings just the same distance each side of the center
of the scale. You now have the required weight of substance ready
for your experiment. Always use a fresh, clean piece of paper for
each weighing.
EXPERIMENT 2 - To weigh a liquid on
your balance
First place a small dish or a beaker on the left pan of the balance.
On the right pan place a dish or bottle which is slightly lighter
and carefully add sand to this dish until it just balances the
other. We call this a counter-balance. You may want to make up
permanent counter-balances using small stoppered bottles with sand,
for the beakers you use most often. Finally place the required
weights on the right pan beside the counter balance and slowly pour
the liquid to be weighed into the beaker until the
34
GILBERT CHEMISTRY
balance pointer again swings an equal distance to right and left of
the center of the scale. With practice you will leam to
recognize when you have nearly the required quantity of liquid and
can add the remaining portion drop by drop to avoid getting too
much. In case a little too much liquid has been poured into
the beaker on the balance, you will find a medicine dropper very
convenient for removing just the right amount.
Always clean the balance pans immediately if you spill either solids
or liquids on them.
EXPERIMENT 3 - To make an
additional 10 gram weight for the balance
Since your balance is rugged enough to weigh far more than 10 grams,
you may wish to make up additional weights to use with it. First
make an additional 10 gram weight. Get a thin walled glass vial
which, with a cork stopper, weighs less than 10 grams. Place the
vial and stopper on the right pan of the balance and your original
10 gram weight on the left pan. Pour grains of sand into the
vial until the pans just balance. Stopper the vial and you have a
practical 10 gram weight. If you wish to label it, the label
should be stuck on to the vial before you bring it to the exact
weight with sand.
Notice that in this experiment you have reversed the usual procedure
and placed the vial on the right pan of the balance and the weights
on the left. This is to correct for any inequality in the lengths of
the balance arms. Ordinarily in using the balance for weighing, the
weights are always
placed on the right hand pan. You can now see that the vial of sand
when placed on the right pan will always balance 10 grams of any
material on the left hand pan. Make two of these 10 gram
weights.
EXPERIMENT 4 - To make a 20 gram
weight
Obtain a glass vial with a stopper weighing less than 20
grams. Place these on the right hand pan of the balance and on
the left place the two 10 gram weights you have just made. Proceed
as in the previous experiment to put sand in the vial until the
balance pointer swings freely an equal distance each side of the
center of the scale, then stopper the vial tightly.
EXPERIMENT 5 - To make a 50 gram
weight
Proceed as above, but place on the left hand pan the two 10-gram and
the 20-gram weights you have made, together with the original 10
grams that came with your set. You may find it better to use small
shot or iron filings instead of sand to give the necessary weight to
the vial.
If you have made all these weights, you will find that you are able
to choose combinations to weigh any article up to 100 grams.
EXPERIMENT 6 - To make a 1-ounce
weight
While you will usually use grams instead of ounces in chemical
experiments, you may wish to make u a set of weights in ounces
(avoirdupois). To do this place on the left pan of the balance a
combination of weights totalling 28 1/2 grams and proceed as before.
THE
THERMOMETER
The thermometer is one of the chemists most useful
instruments. It can be dipped into corrosive liquids without
damaging it. Care should be used in handling the thermometer
as too sudden changes in temperature [may] sometimes break the glass
GILBERT
CHEMISTRY 35
THE
SPATUAL AND MEASURE
One measure of a dry chemical means as much as can be held in the
spoon-shaped measure, Fig. 3. For transferring solid materials
and for rough measuring the chemist uses a flat blade called a
spatula. Your set has been equipped with an improved spatula
having the flat blade at one end and a small spoon-shaped measure at
the other. Even when made of corrosion-resistant metals, a
spatula is soon corroded by by chemicals unless you wash and dry it
immediately after use. A roll of inexpensive paper toweling is
invaluable for this and similar purposes in your laboratory.
THE
MEASURING SPOON
One teaspoonful of a chemical means as much as the spoon will hold
after tapping it lightly. The teaspoon is also used for
heating solids. Fig. 4.
BEAKER
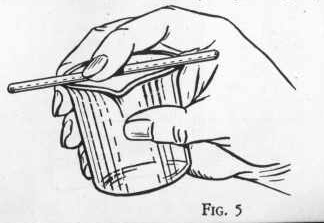
The beaker is a straight sided glass container generally used for
mixing and heating quantities too large for a test tube. It should
be of a quality of glass capable of standing sudden changes without
breaking. Even with the best of glass a wire gauze or an
asbestos mat should be placed between the free flame and the bottom
of the beaker.
Although the beaker is provided with a lip for pouring, liquid
sometimes runs down the outside unless a glass rod is held across
the lip of the beaker in the position shown in the sketch.
36
GILBERT CHEMISTRY
EXPERIMENT 7 - To pour liquid from
a beaker
Fill a beaker quite full with a liquid (water, for practice) and
place a stirring rod across the top so that it rests on the lip of
the beaker an the end extends a little beyond the lip. Now pick up
the beaker with the rod held in position by the first finger as
shown in the sketch and tip it slowly to pour, Notice that the
liquid follows the glass rod and is much less likely to run down the
side of the beaker than when no rod is used in this way.
FLASK
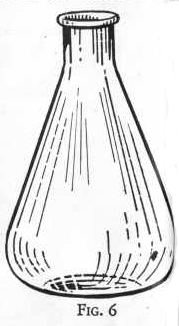
When chemical mixtures are to be heated in such a way as to avoid
the loss of vapors, a flask replaces the beaker. The same good
quality of glass is needed as in the beaker, and the same precaution
to avoid heating over a free flame. By suitable fittings the
flask can be converted into a distilling flask, gas generating
bottle, wash bottle, and many other useful combinations. Never heat
anything in a stoppered flask for it may develop pressure enough
either to blow out the stopper or to burst the flask. In
either case the contents of the flask may be splashed around the
room with bad results.
TEST
TUBES
The test tubes in your set are not the miniature toy affairs
sometimes put into chemistry sets, but practical test tubes made of
especially strong, heat-resisting glass. Some skill is need when
heating liquids in a test tube to avoid sudden explosive formation
of steam which may throw some of the liquid out of the tube
THE
TEST TUBE HOLDER
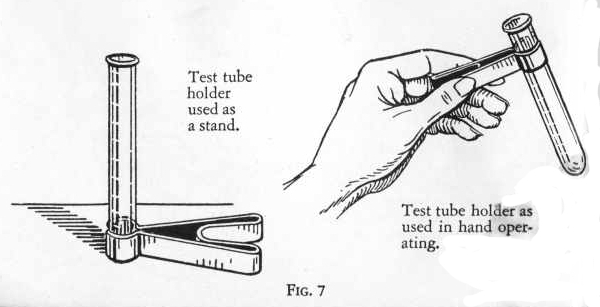
GILBERT CHEMISTRY 37
the Test Tube Holder can be used for two purposes: when
heating mixtures in a test tube it sometimes becomes too hot to hold
with the fingers, and it is recommended to always use the test tube
holder. The Test Tube Holder can also be used as a stand.
THE
STIRRING ROD
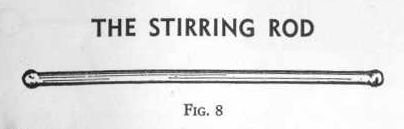
A stirring rod, Fig. 8, is a very convenient piece of apparatus for
mixing a solution when disspoving a solid in liquid. It is a
solid glass rod, round at both ends. Always clean the rod with
water before using it in different solutions.
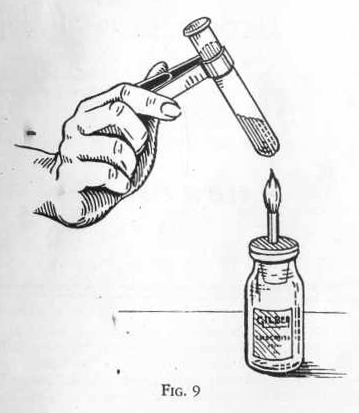
EXPERIMENT 8 - Heating a liquid in
a test tube
Fill a test tube about one~third full with water and attach the test
tube holder near the top of the tube. Hold the tube over the of the
alcohol lamp, keeping it in a slanting position as shown in
the illustration so that the heat strikes the side of the
tube. Maintain a gentle shaking motion to promote smooth and
steady boiling. Even with this precaution, do not point the
open end of the tube toward yourself or any other person while
heating it.
If a test tube has been heated empty or with dry solid materials
inside, do not pour water or any other liquid into the tube until it
has cooled.
38
GILBERT CHEMISTRY
TEST
TUBE BRUSH
A test tube brush has been furnished to help clean the test tubes.
You will find that a little of an ordinary kitchen scouring powder
on the brush will help greatly in cleaning them. Always clean the
test tubes immediately after you are through using them so they will
be clean and dry next time. Clean test tubes are very conveniently
stored upside down on the pegs of your test tube rack.
ALCOHOL
LAMP
Although chemists usually use a gas burner, we have supplied an
alcohol lamp to accommodate the many boys in homes where gas is not
available.
The alcohol lamp supplies enough heat for most laboratory purposes,
and provides a clean flame with less attention to adjustment than
the gas burner. Use a good grade of denatured alcohol. If the
alcohol has been mixed with water, the alcohol will tend to
evaporate first, gradually accumulating water in the lamp until it
does not burn well.
BLOW
PIPE
A more intense flame can be obtained by supplying a jet of air with
the blow pipe as shown in the sketch. This is frequently done in
fusing small lumps of metal compounds on a charcoal block to
identify them, but for longer operations such as glass blowing this
method is awkward and tiresome. Most boys will be tempted to connect
the blow-pipe to some mechanical air compressor such as the vacuum
cleaner.
GAS
DELIVERY TUBE
The gas delivery tube (Figure 12) is used whenever a gas is to be
conducted from a test tube, in which it is formed, into another test
tube or vessel.
GILBERT
CHEMISTRY 39
MORTAR
AND PESTLE
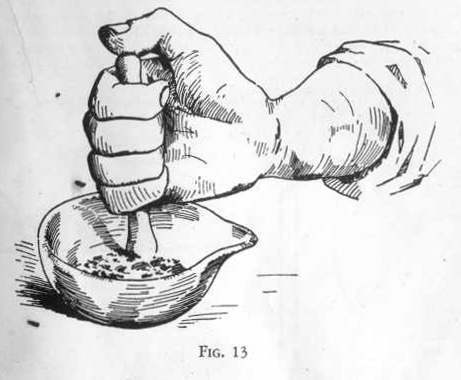
The mortar and pestle is used both for grinding lumps of solids to a
powder and for mixing substances in solid or paste form.
EXPERIMENT 9 - To pulverize a
coarse solid to a fine powder
Place one or two pieces of loaf sugar in your mortar. Now, holding
the mortar in your left hand, take the pestle in the fingers of the
right about as you would hold a pencil in writing. Gently tap
the lumps of sugar a few times until they have crumbled to a loose
mass of small crystals. Never pound hard with the pestle. Next
change your grip on the pestle, holding
it firmly as shown in the picture, and rub the pestle
around the mortar while pressing fairly hard. See how the crystals
of sugar change to a fine white powder.
THE
FUNNEL AND FILTERING
The glass funnel may be used in the usual way to assist in pouring
liquids into bottles, but it is especially designed for filtering.
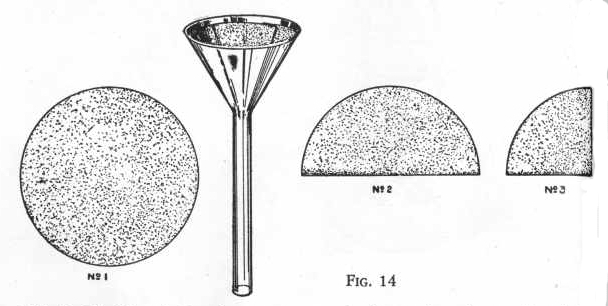
EXPERIMENT 10 - To filter solid
particles from a liquid
For this operation, the funnel must first be lined with filter
paper. The filter paper comes in circles which are to be folded
twice as shown in the drawing, then opened out into the cone-shaped
cup which will fit into the funnel. When a liquid containing
particles of solids is poured into this filter, only the liquid runs
through and all the solid matter is retained on the paper.
You can prepare a suspension of solid particles in water by
dissolving a measure of sodium carbonate in a test tube
one-third full of water and a measure of calcium chloride in the
same quantity of water in another test tube. Now pour the contents
40
GILBERT CHEMISTRY
of one tube into the other and, covering the open end of the tube
with your thumb, mix the contents well. The white solid which forms
is calcium carbonate. Pour the mixture into the filter paper cup
which you have fitted into the funnel. A perfectly clear liquid will
run through the filter, leaving the white solid on the paper.
This liquid, although clear, is not pure water for it contains
substances in solution which cannot be removed by filtering.
THE
GAS GENERATOR
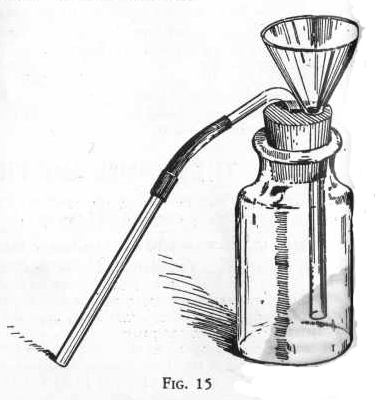
This is a very convenient piece of apparatus for generating and
delivering gases. It is set up as shown in Figure 15.
The end of the funnel in the generator bottle must always be below
the surface of the liquid in the generator bottle, otherwise
some of the gas will go out through the funnel and be lost.
The solid from which the gas is to be obtained is placed in the
bottle and enough water added so that the stem of the funnel will
come just below the surface of the liquid in the bottle. The other
reacting substance, usually an acid, is then added in portions
through the funnel in order to keep up a steady flow of gas.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook