The
Science Notebook
Gilbert Chemistry - Part 3
The
Science Notebook
Gilbert Chemistry - Part 3
NOTE: This book was published in 1936 as a manual
to accompany several Gilbert Chemistry sets of the time.
While some of the experiments and activities here
may be safely done as written, a number of them use chemicals
and methods no longer considered safe. In
addition, much of the information contained in this book about
chemistry and other subjects is outdated and inaccurate.
Therefore, this book is probably best appreciated for
its historical value rather than as a source for current
information and good experiments. If you try
anything here, please understand that you do so at
your own risk. See our Terms of Use.
Pages 41- 60
GILBERT
CHEMISTRY 41
MEASURING
LIQUIDS OR SOLUTIONS
The medicine dropper (Figure 16) is a very useful piece of apparatus
for measuring out several drops of a liquid or solution. The
dropper is filled by pressing on the dropper bulb and inserting the
end of the dropper into the solution. On relieving the
pressure, the dropper is filled with the solution.
Always wash out the dropper with water several times before using it
with different solutions.
GLASS
BLOWING
Only a few simple operations can be undertaken in glass blowing
unless you have special equipment. You should know how to cut
glass tubing and rods, how to fire polish the cut ends, and how to
make very simple bends.
EXPERIMENT 11 - To cut small glass
tubing and rods
To cut a rod or tube, make a scratch by one (and only one) stroke of
a sharp triangular file at the place where it is to be cut. Now hold
the tube in both hands with the thumbs together and pressing gently
on the glass on the opposite side from the scratch. It should
break very easily if the scratch was properly made.
EXPERIMENT 12 - Fire polishing
glass
The edges will be very sharp after a fresh cut, but by holding the
glass over the flame of the alcohol lamp so that only the sharp edge
is in the hot part of the flame you will be able to soften the glass
until a smooth round edge forms. This is called fire polishing.
EXPERIMENT 13 - To bend glass
Cautiously heat the glass in the flame of the alcohol lamp, turning
it continuously so as to be sure you heat it evenly all the way
around. When it has softened, remove the glass from the flame and
bend it a little. Return it to the flame and soften another portion
of the glass just beyond the portion you softened first. Bend the
glass a little more. Repeat, if necessary, until the glass has been
bent to the desired angle.
If you have a gas burner you can heat a larger portion of the glass
at once and you may be able to make the complete bend with only one
heating.
You may find that you can often devise useful a apparatus from
materials that you find around the house. For instance, tumblers and
jelly glasses are just as useful as beakers, provided you never try to heat them.
You can make larger jars by cutting off the necks of bottles or
jugs.
EXPERIMENT 14 - To cut a glass jug
in two
First make a short file scratch at the right height and tie around
the jug at this place a string soaked in alcohol. Light the string
with a match and rotate the jug slowly in a horizontal position
while it burns. The glass usually cracks around under the
string. If it does not, you may help it by touching the hot
glass with a drop of cold water.
[42]
PART II
Inorganic Chemistry and Its Commercial Application to the
Industries
MATTER IN CHEMISTRY
Matter may be either an inorganic or an organic substance. Before
one begins to think in the terms of chemistry, he ought to know
first the difference between materials and immaterial things.
Material things are matter and matter is something that occupies
space; something that takes up room. It can he either inorganic or
organic material. Immaterial things are not matter; for example, a
thought is not matter; it does not take up room; it has neither
length, breadth, nor thickness; you cannot feel, taste or see it. A
good example of a material thing is air enveloping the earth; this
has weight and it takes up room. Water also is matter. In the form
of a small raindrop, it has size, form, and weight; it occupies
space. It is a material thing
KINDS
OF MATTER
Matter is found in different forms such as gas or vapor, liquid and
solid, and many of the same substances of matter may be made to
assume all the three different forms. Water, for example, is
of common occurrence in nature as a liquid, but if the temperature
falls low enough, as in winter weather, water is chilled finally
becomes a solid which we recognize as ice. On the other hand if the
temperature rises high enough, the water becomes invisible and turns
into a gaseous state, which we utilize in the form of steam as a
source of power in the steam engine. Water is an inorganic
substance. The three forms of matter can also prevail among many
organic compounds. For example, if one heats a piece of
camphor gum in an evaporating dish or in a cup, he will observe the
following phenomena: The solid particle of camphor turns to a clear
liquid. In other words, the camphor, when first heated, passes from
a solid to a liquid state by melting. On continued application heat,
the camphor will finally volatilize and become invisible. On
leading the vapors of camphor over a cool surface, the organic
vaporous material will solidify and deposit again as solid
camphor. During all these physical changes there is no
alteration in the chemical composition of the camphor. This same
statement also applies to the inorganic material - water.
DIVISION
OF MATTER
It is possible to divide inorganic and organic matter into very
minute particles. Glass, for example, may be broken up into fine
fragments, and even ground to dust. In the manufacture of Portland
cement we find a typical application of the production of fine
particles of inorganic matter by intensive grinding of mineral
substances. Every single particle of the dust of a pulverized
substance represents the same composition as that of the original
material before grinding. A wild animal running through a forest,
like a fox or deer, may emit odious particles in his travels that
cannot be seen by any human eye, yet the hound can easily pick the
scent and follow them for miles and for hours after the fox or deer
has passed a given point. The odorous principle of a fragrant flower
can be detected by the sense of smell in an atmospheric dilution
corresponding to a percentage of composition that is indeterminable.
GILBERT
CHEMISTRY 43
EXPERIMENT 15 - Division of Matter
Take a pinch of common salt (sodium chloride) and dissolve it in
water. Now taste the solution. You will be able to
detect the characteristic taste of the salt in the solution,
although you cannot see it with the human eye. Now add an equal
volume of pure water to the salt solution. Mix well, and then taste
the solution. You will find that you are still able to discern the
taste of salt. Dilute once more with an equal volume of water and
then taste again the resulting solution. Now you can imagine the
thousands of particles of salt there must be in your water solution
to enable you detect it by taste and yet not to see it.
EXPERIMENT 16 - Division of matter
Take a crystal of potassium permanganate and dissolve it in a
tumbler of water. Observe the coloration of the fluid. The
small particles of the original crystal are now present in all parts
of the water. Their color in solution allows them to be seen. The
performance of this experiment allows you to visualize to what an
extent matter may be divided. Allow a part of the colored
solution to evaporate to dryness and note that the dissolved
permanganate can be recovered in solid form.
EXPERIMENT 17 - Division of matter
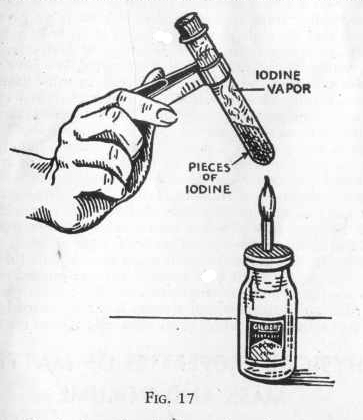
Drop into a clear dry test tube a crystal of iodine which may be
obtained in any drug store and insert a common cork lightly into the
mouth of the test tube (Figure 17), Hold the test tube over an
alcohol or candle lame and warm gently. Watch the beautiful purple
gas that's formed and observe how it creeps, upward toward the mouth
of the test tube. Here you have another demonstration of
extreme divisibility; the solid particles of iodine having been
volatilized by heat and divided into thousands of small gaseous
particles of iodine which are visible to the eye.
44
GILBERT CHEMISTRY
INORGANIC
AND ORGANIC MOLECULES
Molecules are small particles of matter. The three foregoing
experiments that we have asked you to perform have led chemists to
the conclusion that ordinary matter is composed of particles and
that these particles are so small and so minute in size that they
cannot be detected with the human eye or even under the
magnification of the most powerful microscope. These sub-divisions
into minute particles of matter have been designated as molecular in
nature. As we proceed to experimentation in chemistry, we will speak
of these different particles of matter as molecules, and we shall be
instructed in accordance with a law of chemistry, which may be
stated as follows The smallest particle into which matter can be
sub-divided without changing its chemical nature, is a molecule.
Having adopted a designation-"molecule"-to define the smallest
particle of any substance which has all of the properties of the
whole substance, it is now easier to explain the physical difference
between solids, liquids and gases.
MOBILITY
OF MOLECULES IN SOLIDS, LIQUIDS AND GASES
In a solid substance, the chemist conceives molecules as being
firmly held together. They are not free to move about to any
great extent, and are assumed to have had positions. For this reason
a solid substance retains its form or shape. Many substances assume
definite crystalline form of great beauty. In crystals we have
evidence to support the conclusion that the molecules are arranged
in an orderly fashion and that it is due to this orderly arrangement
of the molecules of both inorganic and organic substances that
crystals assume such beautiful symmetry. We have no better
illustration of variations in crystalline form than that revealed by
snow flakes. In other words, the face of a crystal is composed
theoretically of a layer of orderly arranged molecules or lattice
structure of the particular substance under consideration. In a
liquid, on the other hand, the molecules are assumed to be free to
move about among themselves much more freely than is possible in a
solid. For this reason liquids flow and assume the shape of the
receptacle in which they are inclosed. Solids can be fractured by
friction and ground into smaller sub-divisions, while a liquid will
withstand great pressure and cannot be divided by a frictional
force. Gas molecules are assumed to be entirely separate and
mingle with each other and fly apart very widely when they are
allowed to leave the space in which they are confined. In
other words, the molecules are mobile, and due to this property, we
speak of a gas as being volatile and easily diffused. The molecules
of a gas move about much more rapidly than those of a liquid or a
solid. To illustrate, the walls of a football are kept pushed out by
the constant pressure and hammering of millions of air molecules
that are enclosed within the walls of the football. Also the
pressure within a steam boiler is caused by the molecules of water
in the form of water vapor which press constantly against the
sides of the boiler.
PHYSICAL
PROPERTIES OF MATTER MASS AND VOLUME
All matter occupies space. The displacement of space occupied is
called volume. A bushel, a quart, a liter represent, respectively, a
unit of space or a measure of volume of space occupied by a
particular substance. The space occupied by a known weight of
solid, liquid, or gas varies according to the density of the
material. By specific
GILBERT
CHEMISTRY 45
gravity we mean the relation between the volume and the weight of a
substance. Water is used as the standard, that is, 1 cubic
centimeter of water at 4 degrees Centigrade weighs 1 gram. Thus, if
we have 1 cubic centimeter of a substance which weighs 5 grams it
has a specific gravity of 5.
The specific gravity of a substance often enables us to tell whether
or not that that substance has been adulterated. For example,
a certain oil may have a specific gravity of 2. If the
specific gravity when measured is 1.7, for example, the oil has been
adulterated with some other substance.
EXPERIMENT 18 - To demonstrate the
specific gravity of liquids
Place a fresh egg in a glass full of water and notice that it sinks
to the bottom. Now remove the egg and dissolve two spoonfuls of salt
in the water. Then put the egg into the salt solution and notice
that it floats.
The reason why the egg floated in the salt solution was because
we increased the specific gravity of the water beyond that of the
egg. The specific gravity of a solution is always greater than
that of the pure liquid. This is the reason why it is easier to swim
in salt water than in fresh water.
MALLEABILITY
By malleability is meant having the property of being rolled
out or flattened without fracture. This property is
characteristic of many metals. Of all the metals, platinum and gold
are the most malleable, and gold can be rolled into leaves so thin
that it would take 300,000 of these leaves to make the thickness of
one inch. Iron is a very malleable metal when hot, and can be
moulded into many shapes and forms available for human needs. Many
hundreds of men are employed daily in large steel mills in this
country, rolling iron into steel rails for railroads, girders for
buildings and bridges, sheets of metal for roofs, and construction
of oil tanks, and many other applications made possible by the
ingenuity of man.
DUCTILITY
Ductility means having the property of being drawn out into a fine
thread or wire. Iron, copper, platinum, gold, and many other
metals are characterized by this property. Ductile metals find wide
application in industry, especially the electrical industry. It is
interesting to know that the strength of some metals is increased by
drawing them out into small wires and for this reason a drawn wire
is stronger than an ordinary piece of metal of the same dimensions.
Large cables made by twisting iron wire together are very much
stronger than solid iron rods of the same size. Cables of this type
are used in the construction of suspension bridges, of which the
George Washington Bridge extending over the Hudson River, near New
York City, is an excellent example.
BRITTLENESS
By brittleness is meant having the property of crumbling into small
particles when struck a heavy blow with a hammer. Excellent
examples of a few substances which are brittle are ordinary glass,
ivory, chalk, ice, egg shells, and almost all rock.
ELASTICITY
By elasticity is meant having the property of returning to the
original form when it has been changed by some force applied to a
substance. By the return to the orig-
46
GILBERT CHEMISTRY
inal form, the molecules of the substance apparently tend to return
to the places they formerly occupied before the change. Substances
possessing this characteristic property are spoken of as being
elastic substances. The one outstanding illustration of a substance
having this unique property is ordinary rubber. In the stretching of
a piece of rubber we disturb the arrangement of the molecules of
this material.
HARDNESS
A solid is said to be harder than another solid when it will scratch
or make a mark on the other substance. For example, a diamond will
scratch, and even cut glass, because it is harder than glass. You
can cut ordinary metallic lead with a knife blade because it is
softer than the metal of the knife.
FUNDAMENTAL
PHYSICAL CONSTANTS WIDELY USED BY THE CHEMIST
FREEZING
POINT OR MELTING POINT
The melting point of a solid is that temperature at which it is
converted to a liquid. lt is a physical constant which is
characteristic of all substances that are chemically pure. For
example, ice has a melting point of 0° C., or 32° F. above which
temperature it is slowly converted into water. The freezing point of
water (liquid) corresponds to the melting point of ice (solid).
SUPER-COOLING
This is the property of being able to retain the liquid form when
cooled below the temperature of freezing or solidification.
Liquids that assume such a physical state are very susceptible
to outside forces and easily assume a solid form when agitated.
BOILING
POINT
This is the temperature at which a liquid is converted into a vapor.
The constant is dependent upon the atmospheric pressure
existing at the time of distillation of the liquid. For example,
water will boil at a lower temperature at a high altitude, as on a
mountain top, than it will at sea level. In other words, the
boiling point varies according to the variations in barometric
pressure. We have many liquids that cannot be distilled at
ordinary pressure without decomposition, but if such substances are
distilled in a vacuum, they can be easily purified without
destruction. The instrument which is used by the chemist for
determining melting points and boiling points is a thermometer, and
two thermometer scales find application in chemical practice - the
Fahrenheit and the Centigrade scales. According to the Fahrenheit
scale, water freezes at 32° above the zero point, and boils at 212°.
According to the Centigrade scale, the freezing point of water
is 0°, and the boiling point is 100°. In chemical practice and also
for recording scientific data in chemical publications we today make
use of constants as record by both the Centigrade and Fahrenheit
scales.
SUPER-HEATING
Many liquids of high boiling points are very susceptible to
super-heating due to the uneven absorption of heat when the liquid
is being heated to its boiling point. This
GILBERT
CHEMISTRY 47
physical phenomenon is illustrated by the result of rapid heating of
water in a test tube. If heated over a free flame without
agitating the liquid the lower part of the solution will be
over-heated and as a result there will be a sudden evolution of
steam followed by a violent boiling of the fluid. This is referred
to by the chemist as "bumping." Unless precautions are taken
during the heating of liquids, super-heating may result, leading to
serious accidents.
MEASURING
TEMPERATURE
There are two different scales for measuring temperature, the
Fahrenheit scale and the Centigrade scale.
The Fahrenheit scale is most commonly used in our household
thermometers and by the Weather Bureau, but in almost all scientific
work, including chemistry, the Centigrade scale is used. On
the Centigrade scale the point at which water freezes under normal
pressure is marked zero, and the point at which water boils is
marked 100.
The freezing point of water = 0 degrees Centigrade or 32 degrees
Fahrenheit.
The boiling point of water = 100 degrees Centigrade or 212 degrees
Fahrenheit.
1 degree Fahrenheit = five-ninths degrees Centigrade.
To convert Fahrenheit to Centigrade or the reverse use these
formulas:
C degrees = (five-ninths X F degrees = 32) [NOTE: Should read: C degrees = (five-ninths X F degrees -
32)]
F degrees = (nine-fifths X C degrees + 32)
THE
ELEMENTS AND CHEMICAL CHANGE
All of the changes which take place in chemical reactions are based
on the actions of certain substances which the chemist calls
"elements." A boy or girl should, therefore, first acquire the
correct meaning of this term if he or she is to perform
understandingly chemical experiments. By elements the chemist
means those substances which he is not able to break up into simpler
substances. An element may be a solid, such as copper or iron;
a liquid, such as mercury; or a gas, such as oxygen or
hydrogen. It is also important to emphasize here the fact that
some elements can exist in all of the three different physical
forms. For example, ordinary iron is a solid, but will become
a liquid if heated hot enough, or if heated at a very high
temperature, it will become a gas. Mercury can be heated to
form a gas, or it can be cooled to form a solid. The gas
oxygen can be cooled to a liquid: or when cooled still further, to a
solid. In all of these physical changes of iron, mercury and
oxygen, brought about by changes of temperature. we have not altered
the elementary nature of these three substances.
More than ninety of these elements have been discovered to date, and
we know that they combine in millions of different ways to form
every substance that we know of on our earth. When elements
combine with each other they form what the scientist calls
"compounds." When elements are converted into compounds,
they lose their chemical identity and we create new substances
possessing different properties. Such phenomena we explain as
chemical changes, and the chemist speaks of such chemical changes
also as chemical reactions. For example, sulphur is an element
and oxygen is an element. When the sulphur is heated it
attracts to itself the element oxygen, and undergoes a chemical
change. In other words, the sulphur and oxygen undergo a
chemical reaction to form a compound which is a gas - sulphur
dioxide. This gas finds wide commercial application as a
bleaching agent, and also in the manufacture of electric
refrigerators. The study of the elements and compounds, their
properties, their chemical changes, and reactions is called
Chemistry.
48
GILBERT CHEMISTRY
THE
ATOMIC THEORY
Under the heading - Inorganic and Organic Molecules - we have a
definition of the term "molecule," and stated that this is the
smallest particle into which matter can be divided without changing
its chemical nature. This definition of a molecule applies to both
elements and compounds, and just as long as one does not subject an
element or a compound to an experimental condition which leads to a
chemical change or reaction, the substance is said to retain its
original form.
When a chemical change or reaction takes place, the molecules of an
element or compound are destroyed and new molecules are formed.
ln order to interpret such phenomena, it became necessary for
the chemist to originate an imaginary concept or postulation as a
working hypothesis. The result was the creation of a general
law of nature which has been for several years the fundamental basis
of chemical reasoning. This fundamental theory of chemistry is
known as the Atomic Theory. The chemist's explanation of chemical
change or chemical reaction on the basis of the Atomic Theory is as
follows: He assumes that there are particles in matter that
are much smaller than the molecules to which we have previous!
referred. This division of molecules takes place under conditions
favoring a chemical change, and when molecules break up into these
new imaginary particles the substance becomes very unstable and very
reactive. The chemist has named these invisible and imaginary
units "atoms," and when compounds are formed by interaction of
elements or otherwise, it is the atoms that involved, and new
compounds are formed. In other words, molecules of elements or
compounds lose their physical identity when they react chemically to
form new substances, and are converted first into atoms. These atoms
are very unstable and cannot exist alone, and consequently seek
other atoms to make new molecules. The result, in a chemical
sense, is a chemical reaction, with formation of a new substance
having entirely different chemical and physical properties than
those of the original substances brought together.
Therefore, as a result of the above reasoning, chemists have adopted
the law of the atom to guide them in their chemical reasoning. lt is
as follows: An atom is the
smallest particle into which matter is divided in chemical
changes. Chemistry then has a much broader
meaning when we work according to such a theory, and may be defined
as the science of atoms, and how atoms combine with each other to
form compounds.
The interaction of sulphur and oxygen may now be explained as
follows: When these two elements combine with each other it is
not the molecule of sulphur and oxygen that react, but their atoms.
The atoms on being formed cannot exist alone, and attract each
other, leading to the formation of a new compound in which the
properties of sulphur and oxygen have been lost entirely. An atom of
sulphur (S) combines with two atoms of Oxygen (O2) to
form a new substance, or gas (SO2) . The combination of
two different atoms in this case leads to the formation of a new
molecule.
THE
MODERN CONCEPTION OF THE ATOM
Great advances in the science of chemistry and physics have been
made by scientists in the last twenty-five years. New theories
regarding structure have been advanced which have led to a complete
revolution of previous concepts. According to the newer
theories of the structure of matter, the atom is not the smallest
particle which functions in a chemical change, but we now conceive
the existence a still smaller unit than the atom, - namely, the
electron. This new conception of chemical change is electrical in
nature and the electron is the unit of electrical charge. These
newer postulations are constantly undergoing modification and are
far too advanced and theoretical to be comprehended by boys and
girls for whom this manual is written, but it is not
GILBERT
CHEMISTRY 49
out of place to record here some of the conclusions that have been
accepted and which are influencing the development of modern
chemical reasoning. The modern concepts regarding the structure of
atoms may be stated briedy as follows:
1. Each of the 92 elements which we believe to constitute all matter
is made up of atoms.
2. Atoms in turn consist of units which we call protons and
electrons.
3. Protons are units charged positively and electrons are units
charged negatively.
4. Every atom contains an equal number of positive and negative
charged units.
5. The atom owes the greater part of its weight to the positively
charged protons.
6. All of the protons and a part of the electrons of each atom are
concentrated within a small space at the center of the atom. The
remaining electrons are located in layers or "shells" outside of the
nucleus. The nucleus may be conceived therefore as the sun of a
planetary system around which rotate the planets (electrons). [NOTE: # 6 is definitely not considered
correct today. There are protons and neutrons in the nucleus
and electrons in the area surrounding the nucleus. Remember,
this was 1936, and not all of what we know about the atom had been
discovered by that time!]
KINDS
OF CHEMICAL CHANGE
In general, there are four kinds of chemical changes which we need
to consider, and every chemical reaction illustrated in this book
will come under one of these changes. We illustrate by
experiments the four important types of chemical reactions of
elements. First, we have that of Direct Union, or the
combining of two elements to form a compound; secondly, we have what
the chemist refers to as Decomposition or Degradation, which means
the breaking down of a compound into its elements or into simpler
substances; thirdly, what is called Double Decomposition or the
exchange of elements in two or more substances to form new
compounds; and fourthly, Substitution or Replacement, a reaction in
which one element takes the place of another in a compound, the
substituted element being set free.
CHEMICAL
CHANGE THROUGH DEGRADATION BY HEAT
EXPERIMENT 19 - Decomposition of
sugar
Put two measures of granulated or table sugar in a dry test tube and
cautiously heat the tube over an aalcohol lamp. Note the change in
color of the sugar and its tendency to liquify. Watch the
molten sugar and continue heating until it begins to char and turn
black. Here we have a simple demonstration of degradation of
an organic substance by hear. A chemical change has taken
place by heating, leading to destruction of the sugar molecules with
formation of water molecules and ordinary carbon. The identity
of the carbon is disguised in the colorless sugar molecule, but is
revealed when the sugar molecule undergoes decomposition. It
is a very common property of many organic substances to decompose on
heating. All organic animal matter decomposes on intense heating.
DECOMPOSITION
OF AN INORGANIC SALT BY HEATING
EXPERIMENT 20 - Decomposition of
sodium thiosulphate
Place 3 measures of sodium thiosulphate in a clean, dry test tube
and, using a test tube holder so as not to burn the fingers, heat
over the alcohol lamp. You will notice that moisture forms on the
inside of the test tube and some steam will be given off.
Sodium thiosulphate contains water of crystallization which is
driven off in the
50
GILBERT CHEMISTRY
form of steam when the substance is heated. On further heating you
will notice that sulphur is driven of and is deposited on the upper
part-of the test tube. You will recognize the odor of hydrogen
sulphide gas. The material left in the bottom of the tube consists
of sodium sulphate and sodium sulphide. The results of heating may
be represented as follows:
sodium thiosulphate + heat = water
+ sulphur + hydrogen sulphide + sodium sulphate + sodium
sulfide
CHEMICAL
CHANGE BY DOUBLE DECOMPOSITION
EXPERIMENT 21 - Action of ferric
ammonium sulphate on calcium oxide
In a test tube of cold water add 1/2 measure of ferric (iron)
ammonium sulphate. Place the thumb over the mouth of the test
tube and shake to dissolve the solid. Now add 1/2 measure of
powdered calcium oxide and shake again. A reddish brown precipitate
is formed.
The iron of the ferric ammonium sulphate changes places with the
calcium of the calcium oxide to form calcium sulphate and ferric
(iron) hydroxide, which is insoluble in water, and appears as a
reddish-brown precipitate.
EXPERIMENT 22 - Action of aluminum
sulphate on strontium nitrate
Dissolve 1 measure of aluminum sulphate in a test tube 1/4 full of
water. In another test tube 1/4 full of water dissolve 2 measures of
strontium nitrate. Now pour the contents of the second tube into the
first and observe the formation of a white precipitate. The
aluminum of the aluminum sulphate changed places with the strontium
of the strontium nitrate to form the soluble compound aluminum
nitrate, and the white precipitate of strontium sulphate which is
very insoluble in water.
SUBSTITUTION
OR THE DISPLACEMENT OF ONE ELEMENT BY ANOTHER
EXPERIMENT 23 - Action of iron on
copper sulphate
Add 1 measure of copper sulphate to a test tube half full of water.
Close the mouth of the test tube with your thumb and shake until all
the solid is entirely dissolved. This gives a blue solution of
copper sulphate commonly known as blue vitriol.
Now add 1 measure of powdered iron to the copper sulphate solution
and shake the contents of the test tube for a few minutes. The green
color of the copper sulphate solution will gradually disappear and
the iron will become covered with a red deposit of copper.
EXPERIMENT 24 - Action of zinc on
copper sulphate
Repeat the preceding experiment, using powdered zinc in place of the
iron. Notice that practically the same results are obtained.
EXPERIMENT 25 - Action of magnesium
on copper sulphate
Repeat the preceding experiment, using powdered magnesium in place
of the iron. Notice that in this experiment similar results
are obtained.
EXPERIMENT 26 - Action of zinc on
hydrochloric acid
Dissolve 3 measures of sodium bisulphate and 4 measures of ammonium
chloride in a test tube 1/4 full of water. The sodium bisulphate
reacts with the ammonium chloride to form hydrochloric acid which is
soluble in water. Heat cautiously over an alcohol
GILBERT
CHEMISTRY 51
lamp until a clear solution is obtained. Now add to this acid
solution 1 measure of powdered zinc and you will observe an
immediate reaction and bubbles of gas will be generated. This
gas is hydrogen formed by action of zinc on the hydrochloric
acid.
CHEMICAL
CHANGE BY DIRECT UNION OF ELEMENTS
EXPERIMENT 27 - Union of zinc with
sulphur
Both zinc and sulphur are elements. Mix 1 measure of powdered zinc
with an equal amount of sulphur on a piece of white paper. This is
not a chemical compound, for we could readily separate the sulphur
from the zinc mechanically by treating the mixture with carbon
bisulphide which would dissolve the sulphur and leave the zinc
behind.
Place this mixture on the cover of a baking powder can and heat over
the candle flame or alcohol lamp for a few minutes, keeping the face
a safe distance away from the cover until the reaction is apparently
over. You will note that the sulphur and zinc suddenly flash up and
combine. The zinc reacts with the sulphur directly to form a
compound called zinc sulphide. This substance is different from the
original mixture, for if we break this up into a powder and treat it
with carbon bisulphide we cannot dissolve out the sulphur, for it is
now in chemical combination with the zinc.
Break up the zinc sulphide which you have just made into a powder by
grinding in a mortar and put some of this into a dry test tube. Add
one measure of sodium bisulphate, a few drops of water and warm over
a flame for a minute. Remove the test tube from the flame and smell
the gas given off. This is hydrogen sulphide. This is
the gas formed in eggs when they decompose.
EXPERIMENT 28 - Union of magnesium
with oxygen
Place a small amount, only 1/2 measure, of powdered magnesium in the
spoon and and heat it over the alcohol flame, keeping your face at a
safe distance. Notice the sudden flash and the light powdery
substance remaining. The metal magnesium combines with oxygen in the
air to form a white powdery substance called magnesium oxide.
Explanation of the behavior of the metals in the preceding
experiments: The metals may be arranged in what is known as the
electromotive series; that is, in the order in which they dissolve
in acid solution. Metals which dissolve readily, for example, in
sulphuric acid will displace those metals which do not dissolve in
this acid solution or which dissolve only with difficulty. Any metal
will replace any other metal below in the electromotive series,
thus: -
magnesium metal + iron sulphate =
magnesium sulphate + iron
zinc metal + copper sulphate = zinc sulphate + copper
tin metal + silver nitrate = tin nitrate + silver
copper metal + mercury nitrate = copper nitrate + mercury
THE
ELECTRIC CELL
This is the fundamental principle of the Daniell Electric Cell. The
voltage or strength of an electric cell depends upon the diference
between the electrode potentials of the metals used for the two
poles (+ and -) of the battery. For example: a zinc-copper
battery couple gives a greater Electro Motive Force than a zinc-lead
couple or an iron-copper couple. The farther apart the
elements are in the electromotive series the greater will be the
electrical voltage ofthe battery cell.
52
GILBERT CHEMISTRY
ELECTROMOTIVE SERIES
Element
Iron (ferrous)
Tin
Lead
Iron (ferric)
Hydrogen
Copper
Silver
Mercury |
Electrode potential
+0.4410
+0.1360
+0.1220
+0.0450
0.0000
-0.3400
-0.7978
-0.7986 |
AMERICA'S
GENIUS IN THE FIELD OF ELECTRICITY
We often hear the question asked: Did the founders of science make
their contributions before they were thirty or afterwards?
Practically all the pictures that are available of the
different founders of science represent them as old or middle-aged
men. It is true, however, that the founders of science
actually did things before they were thirty. The things that
they did were discoveries made, theories advanced, laws
formulated, classic experiments performed, and methods of
teaching which commanded the attention of the public. The
important things that they did did not receive much attention at the
time they did them, but since their work, they have been given
credit and recognition. Thomas A. Edison began his important work
when he was a very young man, and his invention of the incandescent
lamp was one of his earliest contributions. Mr. Edison was a
ceaseless worker and continued to take an active interest in his
work until his death. Every American boy should be proud of the
record of this wonderful man.
THE
ATMOSPHERE
The air enveloping our earth is essentially a mixture of two
elements - oxygen, which comprises about one-fifth of the air by
volume, and nitrogen, four-fifths volume. Actually, oxygen and
nitrogen comprise 99 per cent of the atmosphere by volume at sea
level, the remaining one per cent being made up of a mixture of
gases, namely argon, helium and neon, mixed with traces of hydrogen
and carbon dioxide. Oxygen is considered to be the most abundant of
all the elements and most widely distributed. Eight-ninths of water
by weight is combined oxygen. Such common materials as sandstone,
quartz, limestone or marble, common brick, granite, clay and cement
contain fully one-half their weight of oxygen. About
two-thirds of the weight of the human body is oxygen. The total
weight oxygen in the land, the water,the atmosphere, and in living
organisms may be regarded as very nearly equal to the combined
weights of all the other elements.
OXYGEN
Oxygen of the air plays a very important role in our everyday life.
We breathe this element into our lungs and from these organs it is
carried by the blood-stream throughout the body to combine with the
waste products of body metabolism. These waste materials are
oxidized by the oxygen, carried in the blood stream throughout the
body to carbon dioxide which is carried back to the lungs by the
blood and is there passed out into the air when we breathe. lt is
very essential, therefore, that we breathe in fresh air if we wish
to enjoy good health. Pure oxygen is a very active element, and if
it were not
GILBERT
CHEMISTRY 53
for the fact that the oxygen of the air is diluted with
nitrogen gas, a very inactive substance, the world would soon burn
up and all living organisms be destroyed.
Oxygen is said to support combustion, but the gas will not burn
itself. A fire will not burn unless air rich in oxygen is constantly
supplied so that the fuel can have plenty of oxygen to support
comustion. Substances cannot burn without oxygen.
OXYGEN
EXPERIMENTS
EXPERIMENT 29 - To remove oxygen
from the air
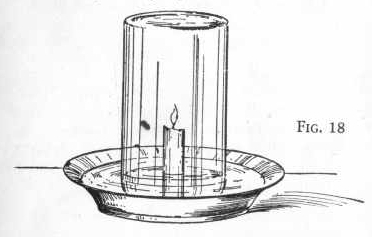
Place a candle in the center of an ordinary wash pan by allowing a
little of the melted wax of the candle to fall on the pan to stick
the candle firmly. Then pour into the pan two inches of water
(Figure 18). Light the candle and place over it a mason or fruit
jar. Be sure that the jar is high enough so that the flame
does not come too close to the top of the jar.
You will notice that very soon the flame grows dim and finally dies
out, the oxygen of the air within the jar having been entirely used
up. Notice that the water begins to rise inside the jar and stands
at a higher level than the Water in the pan. This is because the
oxygen was removed forming a partial vacuum which drew the water up
inside the jar. The oxygen in the jar united with the carbon
in the flame to form carbon dioxide, a gas which dissolves in water,
and with hydrogen to form steam. which condenses in water. The
gas remaining in the jar is nitrogen.
EXPERIMENT 30 - Preparation of
oxygen and how to test for it
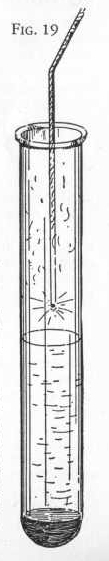
Fill a test tube about half full of ordinary hydrogen peroxide or
dioxygen solution (which may be purchased at a drug store) and add 1
or 2 measures of manganese dioxide. Notice what takes place.
Now light a splinter of wood or string, blow out the flame, being
sure that there is a spark remaining on the wood or string and lower
the spark into the tube above the liquid. (Figure 19). Notice that
the wood or string takes fire and burns with a flame. This is
the way to test for oxygen.
Hydrogen peroxide is a liquid composed of two atoms of hydrogen and
two atoms of oxygen just as water is composed of two atoms of
hydrogen and one atom of oxygen. The manganese dioxide is added to
make the hydrogen peroxide give up part of its oxygen and in doing
so remains unchanged itself. The hydrogen peroxide is decomposed
into water and oxygen. A substance 'ke manganese dioxide which
causes a reaction to take place or speeds up a reaction and at the
same time remains unchanged itself is called a "catalytic agent." We
have many illustrations of catalytic agents or reaction promoters in
chemistry, and many of them find wide commercial applications.
54
GILBERT CHEMISTRY
EXPERIMENT 31 - Preparation of
oxygen from potassium permanganate
Place two measures of potassium permanganate in a clean, dry test
tube and heat over an alcohol lamp. Test for the presence of oxygen
the same way as in the preceding experiment.
many substances like potassium permanganate contain large amounts of
oxygen, part or all of which is liberated on heating.
HYDROGEN
PEROXIDE
Hydrogen peroxide is a chemical which every boy and girl should be
familiar with. It is found in every "First-Aid Cabinet" and is
a good antiseptic. Its antiseptic properties are based on its
containing an extra oxygen atom which it gives up very easily.
It is an unstable compound and sometimes the lowering of the oxygen
content is due to the long time the antiseptic has been kept on the
druggist’s shelf. Sometimes, also this chemical is sold as a
high grade peroxide solution when it actually never contained the
required amount of excess oxygen. Hydrogen peroxide solution should
never be taken by mouth.
EXPERIMENT 32-Behavior of hydrogen
peroxide toward blood
Place a drop of blood on a small watch glass and then pour over the
drop of blood a small quantity of hydrogen peroxide solution. Then
mix the blood with the peroxide solution by stirring with a glass
rod. Notice that the peroxide is decomposed with evolution of
bubbles of oxygen. At the same time the red color of the blood is
lost. The blood is destroyed by the action of the generated
oxygen. Many forms of bacteria are killed by contact with hydrogen
peroxide.
EXPERIMENT 33-A standard test for
hydrogen peroxide
Make a solution of potassium permanganate by dissolving 2 crystals
of the solid salt in water as follows: Fill a test tube 1/4 full of
water and add the crystals to the water. Then close the mouth of the
test tube with the thumb and shake vigorously until the crystals
dissolve.
In a glass one-half full of cold water put 3 drops of the potassium
permanganate solution; mix thoroughly and notice the pink color of
the solution. Now to this solution add 5 drops of the hydrogen
peroxide solution to be tested. If the pink color is destroyed, the
hydrogen peroxide solution may be considered of correct
strength. Both the potassium permanganate and hydrogen
peroxide are destroyed. They cannot exist in the presence of each
other.
OXIDATION
Some compounds contain much oxygen and under suitable conditions
readily give up part or all of their oxygen to other compounds. Such
substances are called by chemists - oxidizing agents. In other words
by oxidation, is meant the union of a substance with oxygen.
During chemical oxidation heat is evolved and sometimes light.
Oxidation is an important chemical process. We obtain heat to warm
our homes in winter, and power to run machines in our
factories by burning (oxidizing) wood, oil and coal. Our
houses are lighted by burning (oxidizing) gas or kerosene, or by
electricity that is generated by machinery run by burning
(oxidizing) fuel. When gasoline is ignited and exploded in the
cylinders of an automobile engine, the gasoline suddenly unites with
the oxygen of the air which has been drawn into the cylinders.
An ordinary house heating furnace is an oxidizing machine, and even
man and all living organisms (animals) are likewise active oxidizing
machines. By means of heat
GILBERT
CHEMISTRY 55
obtained by the process of oxidation metals can be melted. By power
obtained from the process of oxidation our buildings are
refrigerated and water can be frozen to artificial ice. Ice
machines are of common commercial use today, and play an important
part in the preservation of health. ln fact, human life and all
human activities depend upon some form of chemical oxidation and
without oxygen our lives and all life activities of animals and
plants would cease.
REFRIGERATION
AND AIR CONDITIONING
Reversed refrigeration, or the process of heating a house in winter
by means of the same equipment for cooling it in summer, is an idea
which is being carefully investigated today by engineering
concerns interested in the manufacture of air-conditioning
apparatus. In summer, air-conditioning equipment absorbs heat
from inside the house, takes it outside and discards it, just as an
electric refrigerator takes up heatfrom inside the food compartment
and releases it into the room. ln winter the cycle is
reversed. heat is taken up from out doors and brought inside
to warm the house. Coils inside the house which formerly
absorbed heat, become radiators, while the outside coils, instead of
throwing off heat, absorb warmth from the outside air. There are
many problems yet to be solved before reversed refrigeration
equipment is commercially successful, but the principle is
constantly finding new applications and a promising future is ahead
for new and important commercial developments.
CHEMICAL
OXIDATION EXPERIMENTS
EXPERIMENT 34 - The making of
a chemical fire
There are many methods of making "chemical fire." One of these is
dependent upon the fact that glycerine when in contact with solid
potassium permanganate takes up oxygen from the potassium
permanganate so rapidly that intense heat is created. The heat
finally becomes so intense that it causes the glycerine to vaporize
and the vapor finally takes fire, burning with a purple flame. The
color of the flame is due to the metallic particles of potassium,
resulting from the decomposition of the permanganate.
EXPERIMENT 35 - How to make a fire
without a match
Upon an ordinary small tea saucer or a cover of a tin can put just
three drops of glycerine - no
more. Then place on the glycerine about 5 measures of
potassium permanganate crystals. Let stand in a safe place. In a few seconds
the whole mass will begin to smoke, and if the proper
proportions of chemicals have been added, as indicated, the
glycerine will suddenly burst into flame and burn with a blue color.
EXPERIMENT 36 - Fire ink
Place 1/2 spoonful of potassium nitrate in a test tube and add 1/2
inch of water. Warm over the candle for a minute to dissolve all the
material.
Now write with this liquid upon some unglazed or porous paper. using
a clean pen or a small brush. Be sure that the strokes are
heavy and all lines are connecting. After the lines are thoroughly
dry apply a lighted match or better a glowing spark to some of the
writing. Blow out any flame that may result. If properly done,
the spark will travel along the lines where the liquid has been
applied leaving the rest of the paper untouched. The potassium
nitrate is a strong oxidizing agent.
This experiment is very mystifying and when performed in the dark is
quite phenomenal and mysterious. The best results are obtained
by using soft paper, and by making the lines heavy and connecting.
56
GILBERT CHEMISTRY
EXPERIMENT 37 - Oxidation of
metallic zinc
Make a mixture of 10 measures of ammonium nitrate and 1 measure of
ammonium chloride on a metal pan or plate and spread the mixture out
in a thin layer. Now sprinkle over the top of this mixture 5
measures of powdered zinc and allow 1 drop of water to fall into the
mixture.
Notice that the mass soon burns, the oxidation taking place so
rapidly that the zinc takes fire. The oxygen is furnished in the
reaction by the ammonium nitrate, and zinc is converted into zinc
oxide. Metallic iron undergoes a similar change when exposed to air
and the metal burns red due to oxidation. This is spoken of in
common language as “the rusting of iron."
EXPERIMENT 38 - How to make a fuse
A very good fuse can be made by soaking a cotton string in a
solution of potassium nitrate or saltpetre for a few minutes and
then allowing the string to dry. Allow the string to be suspended
before drying. You can time your fuse by using the proper
length of string. The nitrate solution is prepared by dissolving 1/2
spoonful of potassium nitrate in a test tube containing 1/2 inch of
water, and then shaking until all is dissolved.
EXPERIMENT 39 - Oxidation of an
element by means of a nitrate
Heat on your spoon one measure of potassium nitrate until the salt
is molten. Then drop a pinch of sulphur into the molten
potassium nitrate and notice the sudden flash. The sulphur
will be oxidized by the oxygen from the potassium nitrate to form
sulphur dioxide. Note the odor of the burning sulphur. In the
preceding experiment, the burning of the cotton fuse leads to the
formation of carbon dioxide, an odorless gas, while sulphur burns
under similar conditions to give sulphur dioxide, having a
characteristic penetrating odor. '
EXPERIMENT 40 - To allow the
increase in weight upon heating iron in the air
A small bundle of iron wool is counterbalanced on a beam balance. lt
is then held by means of tongs, above an alcohol flame until it
ignites. The iron wool is then removed from the flame until it stops
sparking. The entire process is repeated until all the iron has
completely burned. Upon reweighing, the increase in weight is very
apparent. One must be careful not to burn the wool too rapidly so as
not to lose too much of the iron in the form of sparks.
EXPERIMENT 41 - Oxidation of spices
Mix together 4 parts cinnamon, 3 parts of allspice, and 5 parts of
ground cloves and grind together. Now add 8 parts of potassium
nitrate to the above mixture but do not grind. Place some on a spoon
and warm. Notice the odor - much like perfume. Now ignite this
mixture and you will have a phenomena as wonderful as a 4th of July
night fireworks. A beautiful shower of colored fire will be the
result.
EXPERIMENT 42 - Suffocating a
burning candle
Attach a short piece of a candle to a cork and float this on water
in a tin pan about 2 or 3 inches deep. If the candle is top heavy
fasten a nail or small iron weight to the underside of the cork.
Light the candle and cover it with an inverted fruit jar placed with
its mouth on the base of the pan and under the water surface.
As the candle burns it becomes paler and finally the flame dies out.
You will notice that the water level in the jar is higher than at
the beginning of the experiment. Some of the oxygen of the air
has disappeared to support the combustion of the candle. Close the
mouth of the fruit jar with a sheet of card board and set upright on
the table.
GILBERT
CHEMISTRY 57
EXPERIMENT 43 - Testing for
carbonic acid gas
Insert a lighted taper into the jar from the preceding experiment.
The flame will be extinguished. The carbon dioxide formed by
the burning candle will not support combustion.
EXPERIMENT 44 - Burning sulphur
Repeat the candle experiment using some sulphur. Place the
sulphur on a small tin lid resting on a cork. Ignite the
sulphur and burn under the fruit jar. After the flame is
extinguished then set the fruit jar upright on a table and notice
the color of the gas. Also suspend a moistened blue litmus
paper inside the jar. Sulphur burns to form sulphur dioxide.
Sulphur dioxide is soluble in water, forming an acid. This will turn
the blue litmus paper red.
EXPERIMENT 45 - Burning iron
Collect some fragments of metallic iron and free from all iron rust
in order to obtain a clean metal surface. Tacks and small nuts
and bolts are suitable. Wrap these in a piece of sheet cellophane
and force firmly into the bottom of a medium sized test tube.
Then invert this tube in a pan of water, support it by a clamp and
let stand for several hours. the water will gradually rise to
a higher level in the test tube, showing that air has been used up.
The iron is slowly oxidized in contact with moist air and and is
changed by the oxygen of the air forming iron oxide. Examine the
fragments of iron and note their appearance.
EXPERIMENT 46 - Protecting iron
from oxidation
Repeat the preceding experiment, but use fragments of iron which
have been coated with collodion or some material impervious to moist
air. The water will not rise in the jar. You will observe that
there is very little tendency here for the iron to undergo
oxidation. Paint serves to preserve iron from oxidation and
corrosion.
EXPERIMENT 47 - Oxidation of zinc
Polish a strip of zinc metal and repeat the air oxidation experiment
applied with iron fragments.
EXPERIMENT 48 - Oxidation in the
body
The changes taking place in our body are similar to the preceding
changes of the burning candle and sulphur. We are built up of
complex carbon compounds, some of which contain sulphur. When we
breathe we inhale air through the lungs and here the oxygen of the
air is picked up by the blood and carried where needed in the
body. A burning process actually takes place internally, and
the products of combustion are expelled through the lungs.
Bubble the breath through a glass tube into a test tube of lime
water. What happens? A white precipitate of calcium
carbonate is formed, showing the presence of carbon dioxide.
EXPERIMENT 49 - Combustion of
charcoal
Heat 3 measures of potassium nitrate in a dry test tube over
your alcohol lamp until the salt crystals liquify. Holding tube
vertically then drop.into the molten salt some specks of charcoal.
They will take fire immediately. The nitrate furnished oxygen to
burn carbon.
EXPERIMENT 50 - Burning
sulphur
Repeat the preceding experiment with sulphur. This will burn
in the presence of potassium nitrate, forming sulphur dioxide, which
will be detected by its characteristic odor.
58
GILBERT CHEMISTRY
EXPERIMENT 51-Burning paper
Repeat the above experiment with fragments of dry filter paper
fiber.
EXPERIMENT 52-Oxidizing copper
Insert into some molten potassium nitrate a piece of copper wire.
Note the change on continued heating over your alcohol lamp.
EXPERIMENT 53-Oxidizing iron
Repeat the above experiment using a piece of iron wire.
EXPERIMENT 54-Oxidizing aluminum
Repeat the above experiment using a small piece of aluminum foil or
wire.
EXPERIMENT 55-Oaidizing nickel
Repeat the above experiment using a piece of nickel wire.
EXPERIMENT 56-Oxidizing zinc
Repeat the above experiment using some granules of granulated zinc
metal.
EXPERIMENT 57-Oxidizing silver
Repeat the above experiment using a piece of polished silver metal.
EXPERIMENT 58-Asbestos insulation
Repeat the preceding molten potassium nitrate experiment with some
asbestos fibers.
EXPERIMENT 59-Cotton fiber
Repeat the above experiment with cotton fiber. Which is the
best fire insulating material, asbestos or cotton?
EXPERIMENT 60-Oxidizing sulphur in
copper sulphate
Repeat the preceding experiment by fusing potassium nitrate and then
dropping into the hot fluid crystals of copper sulphate. Observe the
behavior and note whether you actually have an oxidation with
formation of sulphur dioxide.
PROTECTION
AGAINST FIRE
Intensive oxidation of combustible material leads to generation of
heat and, as a final! result, to a conflagration. Cloth, wood, paper
and other substances may be rendered fireproof by treating them with
the proper chemicals. Ammonium chloride or sal ammoniac (NH4Cl)
is a cheap salt which can he used for this purpose. The
article to be fireproofed is dipped into or soaked in a strong
aqueous solution of ammonium chloride and then dried. When such
treated material is heated. the ammonium chloride is decomposed with
liberation of ammonia and hydrochloric acid, and a fire is
prevented, as neither the ammonia or hydrochloric acid will support
combustion. When they are generated they smother the flame and
conflagration is prevented. The curtain and scenery of
theaters and tapestries in public buildings are fireproofed as a
protection against fires. Fireproofed wood finds important
commercial use as a construction material. Such chemically treated
materials cannot be set on fire by sparks or flames. Tin salts
find commercial application in fireproofing.
GILBERT
CHEMISTRY 59
EXPERIMENT 61 - To fireproof cloth
or paper
Take a strip of paper or linen and immerse it in a solution made by
dissolving one teaspoonful of ammonium chloride in a test tube 1/3
full of water. When the paper or linen is dry, try to light it with
a match. You will see that it burns while held in the flame but will
go out just as soon as the flame is removed.
EXPERIMENT 62 - To fireproof wood
Wood is also treated sometimes with a strong ammonium chloride
solution. Another way to fireproof wood is to paint it with
water glass solution.
Holding a match by the head, dip the other end in a sodium silicate
solution (water glass). Allow the coating to dry for twenty
minutes, then light the match. The flames will go out just as soon
as it reaches the portion that has been dipped in the water glass
solution.
EXPERIMENT 63 - Fire-proofing cloth
Cloth or other inflammable substances may be fireproofed by treating
them with chemicals which when heated give off vapors that
smother the flame.
Dissolve 12 measures of ammonium chloride in a test tube 1/3 full of
water. Put a piece of cotton cloth 2 or 3 inches square in the
bottom of a glass and pour the liquid in the test tube over it. Stir
the cloth around until it is wet through, then let it dry and try to
light it with a match. You will find that it will burn while held in
the flame, but just as soon as the match flame is removed it will go
out.
EXPERIMENT 64 - Fireproofing with
sodium tungstate
Cloth, paper, wood and similar inflammable substances may be
fireproofed by treating them with sodium tungstate. This process is
frequently used in connection with the curtains and scenery for
theaters. The woodwork on battleships is also treated in this manner
so that it will not take fire from the explosion of shells.
EXPERIMENT 65 - Fireproofing with
sodium silicate
A sodium silicate solution or water glass is often used for
fireproofing. Wood is frequently fireproofed by means of sodium
silicate. This liquid if applied to paper or cloth will cause it to
become stiff when it dries, so it is not suitable for these
materials.
Hold a match by the head and dip the other end into a solution of
water glass (sodium silicate solution). Let the coating which is
obtained dry about 15 minutes then light the match. There will be no
danger of burning your lingers, for the flame will go out as soon as
it reaches the water glass.
FIRE
EXTINGUISHERS
Since carbon dioxide does not burn nor support combustion, it is
used in fire extinguishers. You are all familiar with the hand fire
extinguisher. Remember the instructions: "To operate, turn upside
down." This apparatus is really no more nor less than a huge siphon,
the kind you get carbonated or seltzer water from.
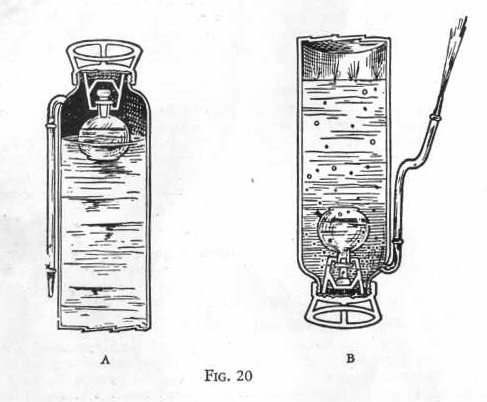
In the large vessel is a dilute solution of sodium carbonate. In the
bottle is strong sulphuric acid. The cork is made of lead and fixed
so that when the tank is turned upside down it falls from the bottle
just enough to allow the sulphuric acid to trickle through slowly.
(Figure 20). (A) shows an interior view of the fire extinguisher:
(B) shows what happens when the extinguisher is turned upside down.
The acid liberates carbon dioxide from the sodium carbonate just as
you liberated carbon dioxide from sodium carbonate with tartaric
acid. The free gas creates a high pressure in the tank, causing
large quantities of gas to dissolve in the water and forcing
60
GILBERT CHEMISTRY
out a stream of gas and water. Carbon dioxide is heavy and surrounds
the flame with a blanket of unburnable gas, which prevents access of
oxygen and in that way smothers the flame.
Quite recently a new style tire extinguisher has come into use. The
container is built like a hand pump. ln the pumplike arrangement is
a liquid called carbon tetrachloride. This is a rather
marvelous liquid. lt voliatilizes, that is, it is converted into a
gas as quickly as alcohol or gasoline but it does not burn. You are
familiar with the explosive power of gasoline. Here is a vapor which
defies all attempts to burn it.
When directed at the base of the flame the spray of carbon
tetrachloride quickly forms a gas which surrounds the burning oil or
wood or whatever it is like a blanket and prevents the access of
oxygen. Without oxygen there can be no combustion, so the fire is
smothered.
Carbon tetrachloride is also a splendid solvent. It is used to
remove grease and paint spots from clothing and to clean white
leather. You have probably noticed advertisements of spot removers
which do not burn. All of these contain carbon tetrachloride.
EXPERIMENT 66 - Carbon
tetrachloride, a fire extinguisher
Pour a little carbon tetrachloride on a piece of paper and light the
paper with a match. Notice that the paper will not burn, proving
that carbon tetrachloride does not support combustion and will not
burn. This is an example of a wet fire extinguisher.
EXPERIMENT 67 - A hand grenade fire
extinguisher
Take 2 test tubes of crude calcium chloride; 2 spoonfuls of common
salt and 1 cup of water and place in thin bottles. In case of fire,
a bottle of this mixture thrown so that it will break near the
flames will put the fire out. This mixture is better and cheaper
than most of the grenades sold for the purpose of fire protection.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook