The
Science Notebook
Gilbert Chemistry - Part 9
The
Science Notebook
Gilbert Chemistry - Part 9
NOTE: This book was published in 1936 as a manual
to accompany several Gilbert Chemistry sets of the time.
While some of the experiments and activities here
may be safely done as written, a number of them use chemicals
and methods no longer considered safe. In
addition, much of the information contained in this book about
chemistry and other subjects is outdated and inaccurate.
Therefore, this book is probably best appreciated for
its historical value rather than as a source for current
information and good experiments. If you try
anything here, please understand that you do so at
your own risk. See our Terms
of
Use.
Pages 161 - 180
GILBERT CHEMISTRY 161
each day taste a little of the cider. You will notice that it
becomes more sour daily, and that finally it will have become unfit
for drinking and has turned into vinegar. This is a well-known
case of fermentation brought about by means of a bacterial
organism. The sweetness of the original cider is due to the
presence of sugar. By the prolonged action of a special low
form of organic life the sugar is transformed into acetic
acid. Test your original cider and the final fermented product
with a blue litmus paper.
EXPERIMENT 528 - Souring of milk
Take a tumbler of sweet milk and set it in a warm place where you
can watch it. Observe the changes that take place day by
day. The milk will become infected with bacteria and undergo
fermentation with formation of lactic acid. This organic acid
is formed from the milk sugar in the fresh milk. The lactic
acid causes the milk to curdle.
EXPERIMENT 529 - Artificial butter
Oleomargarine is an artificial butter made from cotton seed oil and
other vegetable oils. It is a wholesome food product and is
not necessarily inferior to real butter made from cow's milk.
Renovated butter is a process butter made from rancid butter by a
chemical process.
EXPERIMENT 530 - Testing butter
Place a small lump of pure butter in a silver spoon and heat over
your alcohol lamp. Good fresh butter will melt and boil over,
producing at the same time some foam. If it is an
oleomargarine preparation it will sputter and crackle. giving a
sound like that of a burning green stick.
EXPERIMENT 531 - Testing butter
Heat a small pan of sweet milk to boiling. When hot add a
teaspoonful of butter to be tested and stir until melted. Now
pour into a tumbler and cool while stirring. The added butter
will solidify when the milk is cooled to ice temperature.
EXPERIMENT 532 - Testing butter
Repeat the preceding experiment using a sample of artificial
butter. When the milk is cooled the renovated sample will
solidify in a granular condition and be distributed through the milk
in small particles. Oleomargarine will solidify to one cake
and may be lifted from the milk with a spoon or stirring rod.
EXPERIMENT 533 - Milk and the
common cold
Fresh pure milk is a perfect food and contains necessary vitamins
for promoting growth. Faithful use of this food will aid in
protection against the common cold. The true cause of a cold
is not known but it is known that a diet high in vitamin A shortens
a cold's duration and lessens its severity. Therefore, eat
foods rich in this vitamin. Some of these are the following:
milk, butter fat, cream, cheese, eggs, liver, cod-liver oil,
fish liver oils, red salmon, green and yellow
vegetables, fruits, tomatoes and olives.
EXPERIMENT 534 - Changing cider
into vinegar
Put two or three drops of vinegar in a glass full of cider and stir
a few times with a stirring rod. Taste a little of this mixture and
notice that it still has the characteristic cider taste. Now
set it aside in a moderately warm place for a few days and again
taste a little of the cider. After several days you will
notice that the cider has a very decided vinegar taste, having
fermented into vinegar.
The small amount of vinegar which you added furnished the necessary
organisms or ferments which started the fermentation of the cider
into vinegar.
162
GILBERT CHEMISTRY
EXPERIMENT 535-The souring of milk
Set a half glass of milk aside in a warm place for several days and
notice that the milk soon curdles or precipitates out and a mould
forms on the surface of the milk. This is another illustration
of fermentation taking place with the formation of mould.
ESSENTIAL
OILS AND PERFUMES
The use of perfumes dates back as early at 2000 years ago. For
instance, in ancient India the sacred fires in the temples were
perfumed with kusa or kus, the fragrant root of a native
grass. This odor is a constituent of many of the present
popular perfumes as in the azurea bouquet perfumes. In the
early days of China and Egypt perfumes were used in the form of
incense and even today the Chinese still burn large quantities of
this material as a perfume. All the ancient perfumery
materials consisted of crude drugs, flowers, herbs, aromatic gums,
resins and woods of the Orient, also many of the fragrant flowers
which are used in making some perfumes today. Perfumes are
extracted from the crude materials, such as flowers, herbs, etc., in
three different ways. First, by treating the finely ground material
with oil or fats in which the perfume is soluble. Second, by
steam distilling the crude materials during which process the
perfume passes over with the steam. Third, by extracting or
treating the finely ground materials with volatile solvents such as
petroleum ether, carbon tetrachloride or chloroform. The last
process or treating the crude materials with a volatile solvent is
the one most commonly used now.
For many years chemists throughout the world have been devoting much
research to the synthesis or building up of these natural occurring
perfumes until today a new industry has been made possible, the
industry of synthetic perfumes and flavoring materials. These
materials can be made commercially at a much lower cost than when
they are extracted from the natural occurring substances.
Besides this a great many chemical substances have been found which
have most delightful odors and flavors, and which at the present
time do not exist in nature as such. The synthetic materials of
today are successfully utilized in goods of the highest grade and
many odor and flavor effects would be impossible without them.
Let us try making some sweet smelling oils and perfumes from
flowers, fruits and spices.
EXPERIMENT 536 - Making rose water
Fill an iron or tin vessel half full of rose petals and add enough
water to cover the petals. Now place the vessel on the stove and,
when the water starts to boil, cover the vessel with a piece of
absorbent cloth. When the cloth is wet with water from the
condensed steam, remove it and squeeze out the water into a
cup. Repeat this operation five or six times and then
discontinue the boiling. Smell the water in the cup and notice
that it has the sweet odor of the rose.
The rose oil which was originally in the petals volatilized, that
is, passed off with the steam and condensed in the cloth along with
the water. This is the way oil of rose was made in the very
early times. Today rose water is obtained by steam distillation in a
little different manner.
EXPERIMENT 537 - Making geranium
water
Prepare geranium water the same way as you prepared rose water in
the preceding experiment, using the leaves and flowers of the
geranium plant in place of the rose petals. Notice this time
that you have a liquid which has the characteristic geranium odor.
GILBERT
CHEMISTRY 163
EXPERIMENT 538 - Making lilac water
Repeat Experiment 537, using lilac flowers in place of rose
petals. Notice this time that the liquid obtained has the
characteristic lilac odor.
EXPERIMENT 539 - Melting violet
water
You can make violet water the same way as you made rose water in
Experiment 536 by using violet petals in place of the rose petals.
Notice that the liquid obtained has the characteristic violet odor.
EXPERIMENT 540 - How to make sachet
powder
Mix together in a mortar one teaspoonful of cloves, one teaspoonful
of cinnamon, one teaspoonful of allspice and half a teaspoonful of
vanilla extract. Now obtain a cupful of dry geranium or sage
leaves and grind them up into a powder by means of the pestle.
Mix the ground geranium or sage leaves with the mixture of cloves,
cinnamon, allspice and vanilla extract and place the powder thus
formed in a jar with a cover. Notice the fragrant odor
produced from this powder. You can vary the odor of this powder
somewhat by using the dried petals of different flowers.
EXPERIMENT 541 - How to make
incense
Place together in a mortar four measures of cinnamon, three measures
of allspice and five measures of cloves. Grind this mixture by means
of a pestle.
Now pour this mixture on a sheet of paper and mix it with eight
measures of potassium nitrate. Do not grind this
mixture.
Heat a little of this mixture in a spoon over a flame and notice the
fragrant odor given off which resembles that of burning incense.
EXPERIMENT 542 - How to make
wintergreen extract
Place some wintergreen berries in a mortar and grind them up by
means of a pestle. Pour the oil obtained into a test tube and
notice the fragrant odor. Taste a little of the oil and
satisfy yourself that it is oil of wintergreen.
EXPERIMENT 543 - How to make lemon
extract
Remove the skin from a lemon by means of a sharp knife and grind it
up in a mortar in the same way as you did in the preceding
experiment. Pour the oil into a test tube and notice the fragrant
odor. Taste a little of the oil.
EXPERIMENT 544 - How to make orange
extract
Remove the skin from an orange, using a sharp knife, and grind it up
the same as in Experiment 543. Notice the fragrant odor.
Taste a little of this oil.
EXPERIMENT 545 - Potpourri sachet
Mix together in a mortar the following spices or any of them which
you can obtain: Half a teaspoonful of cloves, half a
teaspoonful of allspice, half a teaspoonful of cinnamon and about
half a teaspoonful of extract of vanilla. Grind these
materials together until they are thoroughly mixed.
Now obtain about a quart of dried rose petals and mix this spice
which you have just prepared with the rose petals. When it is
thoroughly mixed you will find that it gives a very sweet, pleasant
odor, and it can be used in sachets or put in jars, thus making rose
jars. Whenever the cover is removed from these jars the sweet
odor will fill the room.
164
GILBERT CHEMISTRY
EXPERIMENT 546 - Sage
One of our common plants which has a very sweet odor is the
sage. The leaves of this plant when dried retain their odor
for a long time, and they can be used to good advantage in sachets
and other places where a sweet smell is desired.
COLLECTED
FROM THE MAILBAG
Experiments with Flowers
EXPERIMENT 547 - Action of ammonia
on a violet
Use a pint fruit jar with a tight cover and place in the bottom of
the jar a mixture of ammonium chloride and calcium oxide to which
several drops of water have just been added. Ammonia will be
generated. Now attach the violet to a string or silk thread
and suspend it in the fruit jar and replace the cover. Note
the change of color in the violet as it remains exposed to the
ammonia gas.
EXPERIMENT 548 -
Repeat experiment 547 suspending different pansy blossoms in ammonia
vapor.
EXPERIMENT 549 -
Ammonia gas and a red rose.
EXPERIMENT 550 -
Ammonia gas and a pink rose.
EXPERIMENT 551 -
Ammonia gas and a red tulip.
EXPERIMENT 552 -
Ammonia gas and petunias of different colors.
EXPERIMENT 553 -
Ammonia gas and red geraniums
EXPERIMENT 554 -
Ammonia gas and red carnations.
PRACTICAL
CHEMISTRY
EXPERIMENT 555 - Heat by chemical
reaction
Place six measures of crystalline sodium acetate which is obtainable
at your store in the bottom of a test tube. Heat the tube over
your alcohol lamp and sodium acetate will melt in its water of
crystallization to form a liquid. Cool, after suspending a
crystal of sodium acetate in the liquid. Crystallization will
set in with evolution of heat. Suspend a thermometer in
the crystallizing fluid and note the rise in temperature.
EXPERIMENT 556-Freezing with
chemicals
Place a glass upon a wooden block and pour water around the glass so
that it stands in water. Fill the glass about three-quarters full of
water and add one teaspoonful of ammonium sulphocyanide or ammonium
nitrate and stir. The glass will soon become frozen in the
block.
GILBERT
CHEMISTRY 165
EXPERIMENT 557 - Glycerine-litharge
cement
Litharge (lead monoxide) which is obtainable in any drug store, is
mixed with glycerine in various proportions depending on the use to
be made of the cement. If a slow setting cement is desired use
pure glycerine. If a quick setting cement is desired, dilute
the glycerine equally with water. This cement is useful for
making water-tight connections between plumbing fixtures and
porcelain. It is very hard when it sets.
EXPERIMENT 558 - Casein-borax glue
Shake some pulverized borax with water until no more dissolves,
using four ounces of water. Now rub casein into a paste with
the borax solution, adding the casein until a thick glue is
formed. Add a little copper sulphate solution as a
preservative. This is useful for repairing wood, porcelain and
glass.
EXPERIMENT 559 - Protecting book
bindings
a. Petroleum jelly is a fine preservative when rubbed into the
covers.
b. Beeswax dissolved in gasoline is also excellent for treatment of
book covers.
Lanolin can also be introduced with the beeswax to great advantage.
EXPERIMENT 560 - Stains for wood
Chemical solutions for staining wood are useful for many purposes in
which a liquid color is wanted.
Chemical
Solution of potassium permanganate
Solution of copper chloride and pyrogallic acid
Potassium ferrocyanide and oxalic acid
Potassium ferrocyanide and ferrous sulphate
Ferric ammonium sulphate and tannic acid
Picric acid |
Color
brown
brown
blue
blue
black
yellow |
TEXTILE
CHEMISTRY AND DYEING
Textile fibers may be grouped in two classes according to their
source: vegetable fibers and animal fibers. Cotton, linen, and rayon
are all of vegetable origin, the first two being the natural fibers
as gotten from the cotton and flax plants, respectively, while rayon
is a very interesting textile material produced by first dissolving
the cotton substance, called cellulose, and then from the viscous
liquid spinning a thin filament which is hardened chemically into a
thread so fine and lustrous that it was originally called artificial
silk. But since its chemical and physical properties were in
many ways unlike true silk, it was soon given a name of its own,
rayon. Cellulose is a common material in the woody structure
of all plants and trees, so it is not necessary to destroy
good cotton fiber to make rayon. It is of further interest
that the solubilized cellulose enters the composition of many of of
our lacquers, and also that, if formed into a sheet instead of line
thread it becomes the well-known cellophane
Silk and wool are produced by animals, the first being the cocoon of
the silk worm, and the other, as you all know, is the warm coat
of a sheep. These fibers are therefore very different from the
cellulose group, and are spoken of chemically as proteins. The
chemical differences between these classes of textile fibers are
important to remember, especially in connection with dyeing and with
spot and stain removal.
The cellulose of vegetable fibers is very easily damaged by strong
acids, but is uninjured by quite severe treatment with alkali.
These fibers do not combine with dyes
166
GILBERT CHEMISTRY
very readily, and it is frequently necessary to deposit the dye on
the fiber in a water-soluble form, then convert it to an insoluble
product, in order to hold it permanently on the fiber.
Animal fibers, on the contrary, are much more resistant to acid and
more easily damaged by alkali than the vegetable fibers. They
combine with many dyes so firmly that the problem of dyeing colors
fast to washing is greatly simplified.
Years ago most dyeing was done with the colored extracts of various
plants. There were a limited number of these and many of the
bright colors so common today could not be obtained.
Gradually there were discovered ways of making new dyes in the
laboratory, building them up from simpler compounds obtained, in
many cases, from coal tar. These dyes, sometimes called
aniline dyes, now supply every hue of the rainbow and have almost
completely displaced the natural dyes.
TESTING
TEXTILE FIBERS
EXPERIMENT 561 - The burning test
Obtain samples of several fabrics. Take a few threads from a
sample and light one end in the flame of your alcohol lamp. If
the sample is an animal fiber it will not burn rapidly but will char
into little black knobs and will produce an odor like burning
feathers or hair. If the threads burn readily, leaving a clean
white ash, they are vegetable fibers. This test is
particularly useful in recognizing whether a sample is silk or
rayon. One type of rayon, cellulose acetate sold under the trade
name "Celanese,” does not burn readily like the other rayons,
but more like silk, except that it does not give an odor like
burning feathers. Hence an additional test is needed if
cellulose acetate is suspected of being present.
EXPERIMENT 562 - Acetone test for
cellulose acetate
Moisten the textile fibers with acetone. Ordinary animal and
vegetable fibers are not changed, but cellulose acetate softens and
may even dissolve completely.
EXPERIMENT 563 - Microscopic
examination
With experience one can soon learn to recognize most of the fibers
by appearance and feel. A magnifying glass or microscope is a
great help. With it, wool is seen to have a rough scaly
surface while silk is quite smooth. Rayon appears much like silk,
but of course the burning test will show them different. The cotton
fiber is seen to he an irregular, flattened tube.
EXPERIMENT 551 - Wetting test to
identify linen
Linen is more readily wet through than the other fibers. This
property is made use of in the following test. Put a small
piece of cloth in a test tube half full of water and add one measure
of sodium bisulphate. Heat the solution to boiling. (Be
sure that the sample is very small so that it does not prevent free
circulation of water.) Take out the sample, wash it
several times with water and dry it. After it is dry, loosen
several of the fibers with a pin or needle and moisten them with a
drop or two of glycerine. Press the cloth between two
blotting papers and then examine very carefully the fibers which
were moistened with glycerine. If they are linen they will be
semi-transparent, while other fibers will appear opaque.
EXPERIMENT 565 - A quantitative
test for fabric composition
The fact that caustic soda dissolves animal fibers without attacking
vegetable fibers serves as a basic for one method of determining
their percentage in a fabric.
GILBERT
CHEMISTRY 167
Put six measures of calcium oxide and six measures of sodium
carbonate in a glass containing two test tubes of water. Boil
for several minutes, then let stand to settle and pour the clear
liquid, which is sodium hydroxide into a clean cup while you wash
the solid residue from the glass. Return the sodium hydroxide
solution to the clean glass.
Now take a sample of cloth which you suspect is made of a mixture of
animal and vegetable fibers such as wool and cotton. Weigh it
carefully, then immerse it in the sodium hydroxide solution and boil
it for a few minutes. This should dissolve any wool or silk.
Remove the remainder of the sample, rinse it in water, dry and
weigh. The loss in weight represents the wool or silk
which had been in the fabric.
DYEING
You will find it very instructive in the following dyeing experiment
if you prepare small patches of cloth containing threads of several
kinds. Cut squares of white cotton, about two inches each way.
Cotton from an old sheet or pillow case is better than new cloth for
by many washings the fibers have become easier to dye. Next
obtain coarse white threads or yarns of wool, silk, rayon, and
linen. With a needle, stitch one of each of these threads
across each square of cotton, using long stitches to keep most of
the thread showing on one side." Trim the threads even with
the edges of the patch.
EXPERIMENT 566 - Dyeing with an
aniline dye
Aniline dyes are now supplied ready for household use, and the
beginner will find these very interesting to experiment with.
You will undoubtedly find some on hand in the laundry.
Dye one of your patches of cloth in one of these dyes, following the
directions on the package. Are all the kinds of textile fiber
in the patch dyed alike?
EXPERIMENT 567 - Using an aniline
dye with a mordant
Dissolve five measures of tannic acid in one test tube of water in a
glass. Soak a patch of cloth in this solution for an hour or
more. Squeeze it out and dry it. Now dye this patch with
the same aniline dye and in the same way as in the experiment
above. When the dyed cloth has dried, compare it with the
previous dyeing. How has the tannic acid influenced the
intensity of color on each fiber?
EXPERIMENT 568 - Dyeing with a
natural vegetable dyestuff
Dissolve three measures of ferric ammonium sulphate in one test tube
of water and soak one of your cloth squares in this solution.
Remove the cloth, press it between layers of paper towel to remove
excess liquid, and allow it to dry.
Next dissolve two measures of tannic acid in one test tube of water
and soak the cloth in this solution. Press out the cloth and dry it
again.
Finally, put three measures of logwood into one test tube of water
and boil it in your beaker until it is a bright red. Put the
cloth into this solution and continue to boil it a short time.
Remove the cloth, wash it well in water, and dry it. What
color have you produced? Are all the fibers dyed equally well?
EXPERIMENT 569 - Another black
dying
Repeat the dyeing as above, but omit the logwood treatment.
Compare the quality of black in the two dyings.
EXPERIMENT 570 - Mordant dying with
cochineal
Dissolve three measures of aluminum sulphate in one test tube of
water in a beaker and soak one of your cloth squares in this
solution. Add two measures of sodium car-
168
GILBERT CHEMISTRY
bonate to the solution, stirring quickly and thoroughly. After a few
minutes remove the cloth, squeeze out the water, and let it dry.
Next put one test tube of water in your beaker, add three measures
of cochineal and boil until the liquor is a dark red. Now put the
cloth in and continue boiling a short time. Remove the cloth,
wash it in clean water and dry it. What color effects have you
produced this time?
Can you dye with other natural coloring matters with or without a
mordant? Try the colors from butternut or black-walnut shucks,
or the extract from chips of osage orange.
EXPERIMENT 571 - How to make e
brown sulphur dye
Put five drops of glycerine in a dry test tube and add one measure
of sulphur and one measure of sodium carbonate. Heat the test
tube over an alcohol or gas flame for several minutes and then allow
the test tube to cool.
Now add a little water to the test tube and allow the test tube to
stand for half an hour until the cake is loosened in the bottom of
the tube. Now pour the contents of the tube into a glass of water
and notice that the dye dissolves and the water is colored brown.
EXPERIMENT 572 - How to make a
black sulphur dye
Mix together on a piece of paper one measure of tannic acid, one
measure of sodium carbonate and one measure of sulphur. Put
this mixture in a clean, dry test tube and heat the tube over an
alcohol or gas flame for four or five minutes.
Now remove the tube from the flame and after it is cool fill the
test tube half full of water and allow the test tube to stand for
half an hour. Now shake the contents of the tube thoroughly
and then pour it into a glass three-quarters full of water. If any
dye remains in the test tube add a little more water, shake again
and pour it into the glass. Notice the dark black color of the
water produced by this dye.
EXPERIMENT 573 - How to make black
logwood dye
Dissolve one measure of ferric ammonium sulphate in a test tube
one-third full of water.
In another test tube half full of water put two measures of logwood
and boil for four or five minutes until the solution is colored a
bright red. Pour this solution into the test tube containing
the solution of ferric ammonium sulphate and notice the black
colored solution which is formed.
EXPERIMENT 574 - How to make dark
red logwood dye
Dissolve one measure of cobalt chloride in a test tube one-third
full of water. In another test tube half full of water put two
measures of logwood and boil this solution for four or five
minutes. Pour this solution into the test tube containing the
solution of cobalt chloride and notice the dark red solution which
is formed.
EXPERIMENT 575 - How to make green
logwood dye
Dissolve one measure of copper sulphate in a test tube one-third
full of water.
In another test tube half full of water put two measures of logwood
and boil this solution for four or five minutes. Pour this
solution into the test tube containing the solution of copper
sulphate and notice the green color which is formed.
EXPERIMENT 576 - How to make blue
horse chestnut dye
Put several chips of the bark from a horse chestnut tree into a test
tube half full of water and boil four or five minutes. Now add a
little household ammonia and let boil again for two or three
minutes. Notice the blue colored solution which is formed.
GILBERT
CHEMISTRY 169
EXPERIMENT 577 - Changing red
logwood solution yellow, then blue
Boil two measures of logwood in a test tube half full of water for
four or five minutes. Then pour this solution into a test tube
containing one measure of sodium bisulphate. Notice that the
solution turns from red to yellow in the presence of an acid.
Now add to this solution two or three measures of sodium carbonate
and notice on shaking the color changes from yellow to reddish~blue
or purple. Red logwood solution is yellow in the presence of an acid
and blue in the presence of an alkali,
EXPERIMENT 578-Changing yellow
turmeric solution brown
If you can obtain a little turmeric dissolve one measure in a test
tube half full of water by boiling the solution for three or
four minutes. Then add one measure of sodium carbonate and notice
that the solution turns brown. Turmeric is yellow in the
presnece of acids and brown in the presence of alkalies.
EXPERIMENT 579 - Changing red
cochineal solution violet, then orange
Put one measure of cochineal in a test tube half full of water and
boil until the solution is bright red. Then add one measure of
tartaric acid to this solution and shake the contents of the test
tube. Notice that the solution turns from red to orange.
Now add two measures of sodium carbonate and shake the contents of
the tube again. Notice that the orange solution now
turns violet.
Red cochineal solution is orange colored in the presence of an acid
and violet in the presence of an alkali.
EXPERIMENT 580 - How to dye cloth
red
Put two measures of ferric ammonium sulphate in a test tube
one-third full of water and shake the tube until all the solid is
dissolved. Now place a small piece of cloth to be dyed in this
solution and after it is thoroughly wet, remove the cloth and allow
it to dry.
Now dissolve two measures of sodium sulphocyanate in a test tube
one-third full of water and place in this solution the dry cloth
which was treated with the ferric ammonium sulphate solution.
Notice that the cloth is dyed red. Remove the cloth from the
solution and allow it to dry.
EXPERIMENT 581 - How to dye cloth
dark blue
Dissolve two measures of sodium ferrocyanide in a test tube
one-third full of water and place in this solution a small piece of
cloth to be dyed. When the cloth is thoroughly wet, remove it
and allow it to dry.
Now dissolve two measures of ferric ammonium sulphate in another
test tube one-third full of water. Place the dry cloth in this
solution. When the cloth is thoroughly wet, remove it and
allow it to dry. This time the cloth is dyed a beautiful dark blue
known as Prussian blue.
EXPERIMENT 582 - How to dye cloth a
light blue
Dissolve three measures of sodium ferrocyanide in a test tube
one-third full of water and place in this solution a small piece of
cloth to be dyed. When the cloth is thoroughly wet, remove it
and allow it to dry.
Now dissolve two measures of ferrous ammonium sulphate in a test
tube one-third full of water. Place the cloth into this
solution and shake the test tube a few times. Remove the cloth
and allow it to dry. This time the cloth will be dyed a light
blue, known as Turnbull's blue.
170
GILBERT CHEMISTRY
EXPERIMENT 583 - How to dye silk
gray
Dissolve two measures of sodium bisulphate in a test tube half full
of water. In another test tube put two measures of logwood and
boil the solution until it is colored bright red. Pour this red
solution into the solution of sodium bisulphate.
Now place a small piece of white silk to be dyed in this solution
and heat the solution to boiling. Remove the silk and notice that it
is dyed gray.
EXPERIMENT 584 - How to dye cotton
iron buff
Make a solution of ferric ammonium sulphate by dissolving two
measures of the compound in a test tube one-third full of water.
Place in this solution a small piece of cotton cloth to be dyed and
shake the contents of the test tube thoroughly. Remove the cloth and
allow it to dry.
Now dissolve two measures of sodium carbonate in a test tube
one-third full of water and place the cloth in this solution. Shake
the contents of the test tube thoroughly and then remove the
cloth. Wash the cloth with water and allow it to dry.
The cloth will be dyed an iron buff. This color is produced by
the precipitation of iron oxide upon the fiber by the alkaline
salt, sodium carbonate.
SPOT
AND STAIN REMOVAL
Chemical treatment is often required in order to remove spots and
stains from fabrics. To do this successfully, you need to know, not
only what chemical will remove the stain, but how well the fabric
will withstand the chemical action. When the fabric is
colored, the problem is still more complicated for the dyes may be
attacked by the chemical. The following general rules
may save you many mistakes.
1. If you use acid in cleaning cotton, be sure it is very thoroughly
washed out before dyeing.
2. Do not use alkali on silk or wool. If possible, avoid the
use of water and clean with a dry-cleaning solvent such as carbon
tetrachloride. Many silks may be washed in water, but you
should first be sure that they have been dyed with colors fast to
washing.
3. Many spot removers act by bleaching with chlorine. Never use
these on silk for chlorine damages silk. Chlorine is also very
likely to attack dyestuffs. If silk must be bleached, use a
solution of hydrogen peroxide made slightly alkaline with sodium
silicate.
EXPERIMENT 585-To remove grease
from clothing
A grease stain which has been in clothing for some time can be
removed by treating the stain with alcohol, gasoline or carbon
tetrachloride. Carbon tetrachloride is the best solvent to
use, as it is non-inflammable, cheap and vaporizes quickly. ln
removing a grease stain in this way, always begin at the edge and
work into the center, rubbing the stain thoroughly with a cloth
containing some of the solvent. This is a case of
solution. A fresh grease spot when removed in this way often
leaves a ring on the clothing. To remove a grease spot place a
little tale or starch over and under the spot and warm the spot with
an iron. The talc will absorb the grease and can be easily
brushed off afterwards. Repeat the process until the spot is
removed. This is a case of absorption.
A grease spot may also be removed by rubbing neutral soap on the
spot until a lather is obtained and then rinsing off with
water. This is a case of detergency.
EXPERIMENT 586-To remove paint from
clothing
If paint is fresh it is not difficult to remove. First rub
turpentine, lard, or linseed
GILBERT
CHEMISTRY 171
oil into the stained spot. Then clean it with carbon
tetrachloride as in the experiment above.
EXPERIMENT 587-To remove ink spots
First rub the spot lightly with a bleaching solution. This is made
by dissolving four measures of calcium hypochlorite in a test tube
half full of water. This will change the spot to a yellow
color. Then pour a little hydrogen dioxide on the spot and again rub
lightly. Notice that the spot is now entirely removed.
This is a case of bleaching. This is a chlorine bleach and
should not be used on silk and only for a short time on wool.
EXPERIMENT 588 - To remove iron
rust
Obtain a small amount of oxalic acid and dissolve four measures in a
test tube half full of water. Rub the spot with some of this
solution and notice that the stain is removed. Dilute
hydrochloric acid can be used in place of the oxalic acid.
Remove the oxalic by washing with water.
EXPERIMENT 589 - How to remove acid
spot
If acid is accidentally spilled on the clothing, pour a little
ammonia on the spots and rub lightly with a cloth. Wash the
spot with water in order to remove the salts that are formed in the
reaction. This is a case of neutralization, the ammonia
neutralizing the acid to form a salt.
EXPERIMENT 590 How to remove alkali
from clothing
If caustic soda is accidentally spilled on the clothing pour some
tartaric acid solution or vinegar on the spot and then wash the spot
out with water. This is another case of neutralization.
EXPERIMENT 591 - How to remove
grass stain
Grass stains may be removed by rubbing the spot with a little
alcohol or carbon tetrachloride.
EXPERIMENT 592 - How to remove
mildew
Dissolve three measures of calcium hypochlorite in a test tube half
full of water and rub the stain lightly with a little of this
solution. Remove the calcium hypochlorite by washing with
water.
EXPERIMENT 593 - How to remove
iodine stain
Dissolve three or four measures of sodium thiosulphate in a test
tube one-third full of water and rub some of this solution on the
fabric stained with iodine. Notice that the blue or brown
stain is quickly removed. Then wash the spot with water to
remove the sodium thiosulphate.
TEXTILES
AND AGRICULTURE
It is a far cry from woolen mittens worn by the country school boy,
and a silk scarf adorning the costume of his sister, to a five
thousand acre sheep ranch in Texas or Australia, and the primitive
industry of reeling raw silk from cocoons in eastern China, but
chemistry is constantly providing new and practical products from
agricultural resources to provide pleasure, comfort and health
in every corner of our country. The boy, who today is
performing his first experiments with a Gilbert Chemistry Set, may
tomorrow be the chemist who will be making life happier and more
secure for nearly every person in our land.
Several attempts have been made to develop silk culture in this
country, but without financial success on account of the high labor
cost. Agricultural experiments organized
172
GILBERT CHEMISTRY
to promote this industry in this country have been inaugurated in
the different states of the Union, from the Atlantic to the Pacific
coasts. At the present time an extensive effort is bein made
to develop a raw silk industry in Southern California. The
worm can apparently be propagated wherever the mulberry tree can be
grown successfully.
SOURCE
OF WOOL
Wool comes from a sheep’s back. So does a lot of dirt and other
materials picked up on his travels. He also exudes from the pores of
his skin oily products which are generated by glands in his body.
Only about one-half of the fleece is actual wool fibre.
Fifteen per cent is grease, 15 per cent salts, and 20 per cent clay
and dirt. Therein lies the reason why the woolen industry
calls on the chemist to advise it how to prepare crude fleece for
public use as clothing. It is a long chemical story from crude
sheep's fleece to a woolen blanket on your bed, to a suit of
clothes, to a pair of warm woolen mittens, and to a dyed rug on the
sitting room door of your home.
EXPERIMENT 594 - Wool scouring
Secure if you can a small quantity of wool fleece before it has been
subjected to any scrubbing treatment. Examine it and try to clean it
by scrubbing with different detergents.
EXPERIMENT 595 - Wool grease
Instead of scouring your fleece with detergents to remove dirt, soak
it for several hours with some petroleum naphtha or ordinary
gasoline. This will remove the valuable grease known in the drug
trade as "lanolin." This wool fat is an important constituent of
cosmetic preparations. Finally apply treatment with detergents
to remove dirt. These two experiments will serve to reveal to
you some of the difficulties facing the woolen manufacturer in
preparing his product for public use.
EXPERIMENT 596 - Clothing
lt takes a sheep and a half to make a man a suit of clothes.
Test the fabric used in your shirt and stockings and determine which
contains wool.
EXPERIMENT 597 - Shoddy
What is the difference between the different forms of woolen cloth -
worsteds, serges, flannel and shoddy? Examine a piece of
shoddy under your microscope. What is there peculiar about its
structure?
SOURCE
OF COTTON
Cotton is a plant product and comes from the cotton plant. It is a
cleaner commercial product than wool fleece and does not offer as
many difficulties in purification. This plant is cultivated in
all parts of the temperate and torrid zones, and is the source of
enormous industries spread over our globe.
EXPERIMENT 598 - Cotton industries
Try to enumerate thirty commercial products which are manufactured
from cotton. Make a chart showing the industries producing
these products.
EXPERIMENT 599 - Cotton plant
Obtain if possible some raw cotton and examine it. Who
invented the cotton gin? What is this machine used for?
GILBERT
CHEMISTRY 173
SOURCE
OF SILK
Silk is an animal product and is produced by the silk worm known as
Bombyx Mori. This worm is cultivated and reared chiefly in
China, Japan and Italy. The silk worm feeds on the leaves of
the mulberry tree, and stores up a liquid silk solution in his
body. After the worm has reached maturity it then exudes this
silk solution in the form of a fine thread and weaves a
cocoon. This cocoon is made u chiefly of a protein
called fibroin and
this is the basis of natural silk. Artificial silk, of which
rayon is an example, is a modification of ordinary cotton.
EXPERIMENT 600 - Cocoons
Secure a silk cocoon and cut it into halves by means of a sharp
knife. Observe the dry chrysalis of the original worm encased
in the cocoon.
EXPERIMENT 601 - Degumming
Take the severed cocoon and boil it for a long time in hot soap
solution. Wash with hot water and then dry the fiber and
note its silky appearance.
EXAMINATION
OF FABRICS WITH THE MICROSCOPE
EXPERIMENT 602 - Wool fiber
Under a microscope wool consists of regular cylindrical fibers
covered with scales. Make an examination of a wool fiber with
your own microscope.
EXPERIMENT 603 - Cotton fiber
Cotton fiber looks like a collapsed rubber tube, twisted on its
axis. Make an examination of a cotton fiber with your own
microscope.
CHEMICAL
IDENTIFICATION OF TEXTILE FIBERS
In the home will usually be found some form of commercial
lyes. These consist essentially of sodium hydroxide and can be
used for the following fabric alkali tests. A tablespoonful of
lye to a teacup of water will give a solution of sufficient strength
for performing these fabric experiments calling for use of alkali.
EXPERIMENT 604 - Identification of
wool
A fiber of wool, when burned, is consumed slowly, a ball-like end
being formed. The odor is characteristic of burning animal
matter.
EXPERIMENT 605 - Solubility of wool
in alkali
Wool protein is dissolved by being boiled with strong sodium
hydroxide solution for a few minutes. Add to an alkaline
solution of wool a few drops of copper sulphate solution.
Black copper sulphide will be formed, showing the presence of
sulphur in wool.
EXPERIMENT 606 - Identification of
cotton
Burn a fiber of cotton and note that it burns quickly without giving
any animal-like odor. The odor resembles that of burning
paper.
174
GILBERT CHEMISTRY
EXPERIMENT 607 - Action of alkali
on cotton
Boil a fiber of cotton with strong sodium hydroxide solution.
Note that unlike wool it will not dissolve.
EXPERIMENT 608 - Action of alkali
on silk
Dissolve a fiber of silk in boiling sodium hydroxide solution; cool
and then add three or four drops of copper sulphate solution. A
lavender coloration will be produced. This is characteristic
of proteins which do not contain sulphur.
EXPERIMENT 609 - Action of alkali
and copper sulphate on cotton
Boil a fiber of cotton with alkali; then cool and add three or
four drops of copper sulphate. No lavender or blue coloration
will be produced. Cotton does not respond to the test first,
because it is not soluble in the sodium hydroxide solution, and
secondly, because it is not a protein.
THE
CHEMISTRY OF THE BODY
Man is dependent on the plant kingdom for his source of food. ln
this way man utilizes indirectly the energy of the sun. Plants grow
up, wither and fall to the ground. They decompose largely into
carbon dioxide and water vapor, which are taken up by new
plant life. The nitrogen is converted into nitrates by bacteria in
the soil, and the mineral matter helps to form new plant life.
Sugars are formed in plant life by the condensation of formaldehyde
which is produced by the action of carbon dioxide on water with the
aid of sunlight. The sugars are then changed into starch by
plant ferments or enzymes and these starches are used by animals to
support life. In animals these starches are changed back into
carbon dioxide and water and the whole process repeats itself.
Up to date, man has acquired through research a very extensive
knowledge of the chemical changes taking place in this marvelous
life cycle.
In the laboratory we have great difficulty in changing carbon
dioxide into formaldehyde and formaldehyde into sugar. In
plant life this is done at relatively low temperature which is less
than that of the body (98.6 degrees Fahrenheit or 38 degrees
Centigrade). This is accomplished by the action of the plant
enzymes and is a very remarkable and important process.
In animal life we find enzymes similar to those in plant life.
Sugar (dextrose) for example, is changed into starch (glycogen) in
the liver and this is later turned into dextrose again in the blood
by the action of another enzyme. The dextrose in the blood is
converted by still further enzymic action into carbon dioxide and
water vapor which are carried to the lungs and passed out on
breathing. This change takes places. in the muscle cells and
furnishes the body heat.
The lean flesh or tissues of our bodies consists chiefly of protein.
The fat is very similar to other animal fats. The bones consist
chiefly of calcium phosphate and chrondrin which forms gelatin on
boiling. In old people there is more calcium phosphate present
and less chrondrin than in younger people, consequently the bones of
older people are apt to be more brittle.
The skin consists of toughened protein matter. The hair and
nails consist also of protein material and are called
keratin. Alkalies attack the skin, hair and nails because
proteins are decomposed by alkali. Mild acids, however, have
little or no effect on these organisms. The perspiration which
comes from the skin contains water, fats, acids and a little
urea. We breathe to a very slight extent through the minute
pores in the skin. This seems to be an important and necessary
process so that it is essential to
GILBERT
CHEMISTRY 175
keep these pores open by bathing, since they are continually closed
by fat which condenses in the perspiration.
The blood, which is the most vital part of the body, is composed of
water, containing in solution, protein material called fibrinogen,
seroglobulin, seralbumin and saline water. Also suspended in
this solution are red and white corpuscles. Neutral salts when taken
into the body are decomposed, the acid constituent usually
hydrochloric acid from salt, going to the gastric juice in the
stomach and the alkaline constituent going to the blood.
Therefore, the blood is normally alkaline. When blood is
exposed to the air it clots. This is due to the fibrinogen in
the blood changing to an insoluble compound called fibrin. The
blood usually contains about 0.1 per cent of dextrose. The red
corpuscles of the blood contain what is known as haemoglobin.
This is an enzyme containing iron. It produces some of the
very fundamental changes in the body in the same way that
chlorophyll does in the plant. This haemoglobin has the
property of taking up oxygen from the lungs and later giving up this
oxygen during the oxidation of the tissues. During this
oxidation heat and energy are formed. The white corpuscles
sometimes called leucocytes are really the policemen in the blood
for they tend to ward off sickness by destroying the impurities in
the blood such as bacteria. When the number of bacteria
becomes too great for these white corpuscles we become sick and then
we have to resort to medicine to help the action of the white
corpuscles.
A very important constituent of the brain and nerve cells is the
substance known as lecithin. This is a complex organic
compound containing phosphorus. Ordinarily the foods we eat
contain small amounts of this substance, thereby furnishing the body
with the necessary amount.
You may ask the question, Why is the blood normally alkaline?
This is easily explained by the fact that it is up to the blood to
remove the acidic carbon dioxide gas from the body. In an
alkaline condition the blood can easily absorb these waste products
of oxidation and in so doing forms sodium bicarbonate. The
sodium bicarbonate in the blood gives up carbon dioxide and water
vapor in the lungs. This leaves sodium carbonate which is the
alkaline constituent of the blood and the blood is now ready to pass
again through the body and take up more carbon dioxide.
The teeth differ from the other parts of the body in that they are
more resistant to chemical action, namely, that of acids. The
mouth of a normal person is alkaline and in this state the teeth are
well protected. Decayed matter if allowed to cling to the
teeth is converted into acids which slowly but effectually remove
the enamel from the teeth and cause them to decay. The enamel
of the teeth is composed chiefly of calcium fluoride. You can
readily see, therefore, that it is very important to brush the teeth
every day and the use of a mild antiseptic as a mouth wash is very
effectual.
It is necessary to say at this time a few words in regard to body
health. There are several influences which tend to keep up a
healthy normal body. These are exercise, fresh air, cheerfulness,
cleanliness, sunlight, pure food and pure water. The
relationship between chemistry and exercise may be shown if we
consider that many chemical reactions take place much more rapidly
and with better results if the solution in which the reaction is
taking place is heated or stirred continually. Just so in the
body. By exercising the body the blood moves faster, the stomach
digests our food better, we breathe much more efficiently and
as a consequence the waste products of the body are more effectually
eliminated and we feel much better.
Sunlight has several effects on the body. It is a powerful germicide
and will kill many organisms which are the cause of disease.
It also acts as a blood stimulator. You probably have noticed
how red the body becomes in the summer time when exposed to the
sun's rays.-
176
GILBERT CHEMISTRY
Cleanliness is a very important contribution toward health.
Dirt is a very good medium or culture for bacteria, so that it is
quite necessary to keep the body clean. We have always spoken
of the importance of keeping the pores of the skin open so that
impurities can come out in the form of perspiration and that pores
may breathe in a certain amount of fresh air. Chemistry has
contributed toward this influence on the health of the body by
furnishing such substances as borax, soap, soda, ammonia,
antiseptics and synthetic remedies.
Pure fresh water is a very important factor in everyday life.
Natural waters contain small amounts of mineral salts, principally
sodium chloride. These are not injurious to the health but rather
beneficial. Waters, however, are apt sometimes to contain
bacteria which may be very injurious to the health. If a water
is suspected as containing bacteria it should be boiled. On a
commercial scale water is purified by several processes, namely
treating the water with ozone, bleachin powder, chlorine,
ultra-violet light or by distillation. Any of these methods
are good if used under the right conditions.
The subject of fresh air is an old one, but one which a great many
people disregard. It is very important that the lungs obtain
the normal amount of oxygen necessary in the oxidation processes
which take place in the body. The effect of poisonous gases when
breathed into the lungs was very ably demonstrated during the great
war. Impurities in the air such as poisonous gases, etc., have
a very remarkable affinity for the blood. They react with the
blood to form much more stable compounds than oxygen does.
Consequently the blood cannot take up the normal amount of oxygen
required in the body, and as a result the blood becomes congested.
In the case of extreme poisoning the lungs become so congested that
we are unable to breathe further, and death follows. In a
poorly ventilated room the carbon dioxide given off from the lungs
soon becomes in excess of the oxygen present in the room, with the
result that we become drowsy or sleepy. This effect seems to be due
to a lowering of the oxygen content in the room and also to a rise
in temperature caused by the body heat.
Cheerfulness is not directly related to chemistry but is mentioned
here because of the influence it has on the health of the body. A
person who is cheerful, particularly during the time of eating, is
apt to be less troubled with indigestion than a person who is
constantly worrying. Loss of appetite is quite often due to
just this thing. Indigestion and loss of appetite are
controlled to a large degree by the nerves, which are apt to break
down in a person who is not cheerful.
The truth of the old saying that an apple a day keeps the doctor
away is now vouched for by science. Apples contain vitamin C, which
has been found necessary and valuable in promoting health and
protection from diseases like scurvy. All nutrition
authorities now report that apples, while they do not contain as
much of this vitamin as tomatoes or oranges, do supply an important
amount of it, particularly if eaten raw, skin and all.
Let us try a few chemical tests to see if we can learn something
about the body.
EXPERIMENT 610 - How to test for
sugar in urine
The excretion of urine from a healthy person should be free from
sugar. If sugar is found in the urine it is an indication that
the liver is not functioning properly and the person has what is
known as diabetes.
Put three measures of cream of tartar baking powder in a test tube
half full of water. After the reaction stops add one measure
of copper sulphate and shake the contents of the tube
thoroughly. Now add four measures of sodium carbonate and four
measures of calcium oxide. Shake the test tube again
thoroughly and filter the contents of the tube, catching the liquid
in another test tube. This clear liquid is known as Fehling's
solution.
GILBERT
CHEMISTRY 177
Now to a test tube one-third full of this solution add four or five
drops of urine and heat the test tube over an alcohol flame nearly
to boiling. Now remove the tube from the flame and see if a
red precipitate has formed in the test tube. A red precipitate
formed in this way is a test for sugar in the urine. The sugar
reduces the copper hydroxide formed in the reaction of red cuprous
oxide. By this test it is also possible to determine the
quantity of sugar in the urine.
Repeat this experiment, using four or five drops of a sugar solution
in place of the urine. The sugar solution is made by
dissolving three or four measures of sugar in a test tube one-third
full of water. Notice the red precipitate of cuprous oxide
which is formed by the action of the sugar upon Fehling's solution.
EXPERIMENT 611 - How to test for
albumin in urine
The presence of albumin may be detected in urine as follows:
Heat a test tube one-third full of the urine to be tested over an
alcohol flame with constant shaking. Do not heat above 175
degrees. This can be told by removing the test tube from the
flame occasionally and feeling of the test tube with the hand.
When the test tube is too hot to hold in the hand the temperature of
the urine is above the temperature required. Do not heat the
urine to boiling. Now remove the test tube from the flame and
allow it to cool. Examine the contents of the test tube.
If a precipitate separates out the urine contains albumin.
Repeat this experiment, using a test tube one-third full of the
white of an egg in place of the urine. Notice that a
gelatinous precipitate settles out. This is due to the coagulation
or precipitation of the albuminous material contained in the white
of the egg.
Albumin in the urine indicates that the stomach is not working
properly, that the albumins are not changed during the process of
digestion. They enter the blood in these forms and are passed out in
the urine through the kidneys.
EXPERIMENT 612 - How to test for
proteins in urine
Dissolve three measures of sodium carbonate and three measures of
calcium oxide in a test tube one-third full of water. Put the
thumb over the mouth of the test tube and shake the contents of the
tube thoroughly. Allow the test tube to stand until the solid
materials settle to the bottom. Now pour the clear liquid into
another test tube. This is a strong solution of sodium
hydroxide.
Now dissolve one measure of copper sulphate in a test tube one-third
full of water.
To a test tube one-quarter full of the urine to be tested add the
sodium hydroxide solution prepared above. Shake the contents
of the tube thoroughly and then add two drops of copper sulphate
solution. The formation of a violet color on shaking indicates the
presence of proteins in the urine. The purple color becomes
deeper on heating the solution to boiling. This reaction is
known as the Biuret Test for proteins.
EXPERIMENT 613 - Testing urine for
acidity
Drop a small piece of blue litmus paper in a test tube one-third
full of urine and notice that the litmus paper turns red. This
proves that urine is generally acidic. This is due to the
presence of sodium dihydrogen phosphate in the urine. The
acidity of the urine depends upon the kinds of food taken into the
body. Vegetable foods tend to decrease the acid content and
even render the urine alkaline.
EXPERIMENT 614 - Testing urine for
ammonia
Put five measures of calcium oxide in a test tube one-third full of
urine and heat the mixture over a flame for several minutes.
Remove the test tube from the flame and smell cautiously at the
mouth of the tube. Do you recognize the odor of ammonia?
Ammonia occurs in urine chiefly in the form of organic compounds
known as urea and creatinine.
178
GILBERT CHEMISTRY
EXPERIMENT 615 - Testing urine for
phosphate
Dissolve one measure of calcium chloride in a test tube one-quarter
full of water.
Add a little of this solution to a test tube one-third full of urine
and notice the formation of a white precipitate. If the urine
is alkaline the precipitate may be due to a mixture of calcium
phosphate and calcium carbonate. When urine is alkaline it
contains, besides small amounts of ammonia, the carbonates of soda
and ammonia.
EXPERIMENT 616 - How to test for
acid mouth
Moisten a small piece of blue litmus paper with the tongue and see
if the litmus paper turns red. If it does the mouth is acid.
Acid mouth is usually caused by an upset stomach or by decayed
teeth. Normally the mouth should be neutral or only slightly
alkaline.
THE
CHEMISTRY OF PLANTS - AGRICULTURE
As our knowledge of the chemistry of the processes of living
organisms - animals and plants - increases it becomes increasingly
difficult to define the boundaries between the animate (living
organisms) and inanimate (inorganic or non-living.) The
significance of this statement will be apparent to any intelligent
boy if he will only stop to consider the dependence of our
civilization on the successful practice of agriculture. The
primitive source of the food of man is the soil, and consequently
the successful practice of agricultural science is necessary if man
is to secure food for his existence. The farmer occupies,
therefore, a most important place in human economy. The energy
which is necessary to promote the different chemical changes
involved in animal and plant growth is derived from the sun.
Deprived of this source of energy, plants would not be able to grow,
agriculture would be destroyed, and man would die.
THE
CHEMISTRY OF FERTILIZERS - FARMING
Did you know that chemistry plays such an important part in farming
and in the fertilization of soil? No doubt you have often seen
the farmer cover his soil in the Spring with manure or other forms
of fertilizers and later work them into his soil. You probably
asked the question, why are these fertilizers put into the soil, and
found that they make crops grow better. That is true, the
crops do grow better. But why is this so? By repeated
experiments it has been found out that there are certain substances
which are very essential to plant growth. We have already
learned how plant compounds, such as starch, are built up in the
plant by means of carbon dioxide and moisture in the air with the
aid of the sun's rays. On the other hand there are other important
substances which are taken up by the plant in the soil to form
complex compounds. These are potassium, nitrogen and
phosphoric acid. These substances must be introduced into the
soil in the form of their soluble compounds as they are taken up by
the plant roots by means of absorption.
Nature plays s a very important part in the formation of nitrogenous
substances in the soil. You probably do not know that in most
fertile soils there exists millions of bacteria which have the
property of converting decayed nitrogenous organic matter into
nitrates which are taken up by the plants. Now it is very
important in farming to see that the soil is kept in a condition
whereby these bacteria are able to thrive in order to keep the soil
fertile. Soils which contain large amounts of decayed organic
matter are kept in a moist condition so that the air does not get at
them are quite often apt to be acidic. The nitrate forming bacteria
are not able to live in soils which are acid so that it is very
important to see that these acids are destroyed. Acid soils also
render other fertilizing substances, such as phosphates, insoluble
or in a condition such that
GILBERT
CHEMISTRY 179
the plant is unable to absorb them in the ordinary process of plant
growth. This acidic condition of the soil is generally
destroyed by neutralization of the acids with lime.
In the ordinary process of farming, since the plants are continually
removing the nitrogen, potassium and phosphorus compounds from the
soil, it is necessary to replenish these substances with fertilizing
material. Now the amounts and kinds of fertilizer to be put into a
certain grade of soil depends upon the condition of the soil and
kinds of crops to be raised. One class of plants will require more
phosphorus than another class. Many formulas have been worked
out including the kinds and amounts of fertilizing substances to be
used for different kinds of plants.
Soils are fertilized in four ways, namely, by decayed vegetable
matter, by rotation of crops, by animal manure and by artificial or
commercial fertilizers.
Decayed vegetable matter such as leaves, grass, etc., is often
worked into the soil as a natural fertilizer, but since they contain
only small amounts of essential fertilizing substances, their use
alone would be inadequate.
The second method of fertilization, namely, the rotation of crops is
widely used in farming and consists in planting fertile land one
season with wheat or other crops and the following season with
cow-peas or alfalfa. These latter plants have the property of
converting nitrogen from the air into soluble nitrates which are
restored to the soil to take the place of that used up by the corn,
wheat and other crops in the preceding season. The conversion
of the air into nitrates is accomplished by certain germs or
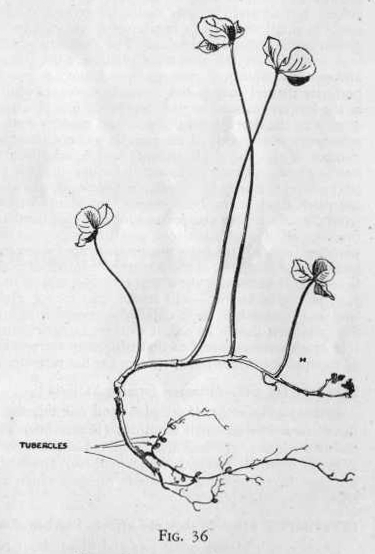
bacteria which occur in tubercles or swellings on the roots of
clover, cow-peas. and alfalfa plants.
Natural or animal manures are used exclusively as fertilizing
materials as they contain large amounts of nitrogen compounds.
They also tend to keep the soils loose so that the air can penetrate
them, Guano, cow and sheep dung are important natural manures
containing relatively large amounts of nitrogen. Guano is the
excrement of sea-birds and is found off the coast of Peru.
Guano is rich in phosphorus, nitrogen and potash.
Artificial fertilizers are used very widely today and several
important industries are involved in their manufacture. These
are chemical compounds or substances that are
180
GILBERT CHEMISTRY
rich in nitrogen, phosphorus and potassium. Phosphorus is
used in these fertilizers in the form of its soluble
compound such as calcium acid phosphate. Potassium is
used principally in the form of potassium chloride, potassium
sulphate and potassium carbonate, while nitrogen is used in the form
of ammonium nitrate and sodium nitrate. The commercial
fertilizers which are put out on the market are complete
fertilizers, such as dissolved potash and phosphates, wood ashes,
ground bones, dissolved bones tankage, dry ground fish, nitrate of
soda, dried blood, cottonseed meal, linseed meal and castor pomace.
One of the important problems of the science of agriculture is to
find out which of the elements that occur in plants and animals are
necessary for their growth. Hitherto the farmer has been taught that
only ten chemical elements are necessary for the growth and normal
support of his crops, It has, however, been a well-known fact
among agricultural chemists that a far greater number of chemical
elements than ten occur in small amounts in fertile soils, and also
in the ashes of plants that have been grown under natural
conditions. The elements which have been regarded as the only
ones essential for the growth of plants are the following: carbon,
hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium,
magnesium, sulphur, and iron. It was perfectly natural that in
the early days of agriculture these elements which occurred in the
largest amounts would receive the first attention by agricultural
chemists. However, with the development of new and modern methods of
chemistry and with greater refinement in methods of research, it was
not unreasonable to expect that a very large number of the chemical
elements which have been regarded as non-essential would finally
prove to be very important factors in plant growth. This has
really proven to be the case. It is now known that the
element, manganese, for example, is a very essential plant
food. It has been shown that plants will assimilate manganese
compounds from the soil. It now also seems probable that manganese
actually exists in small quantities in all living organisms, and has
important functions that have hitherto been unrecognized. All
the facts to date show that manganese is an important and necessary
factor in the synthesis of the green chlorophyll of plants, and
experiments have already been carried out which show that no other
one of the common elements - iron, copper zinc, boron, or arsenic -
will replace manganese. Heretofore, it has been believed that iron
and magnesium were chiefly concerned in the synthesis of plant
chlorophyll. lt has now been shown by recent workers in
agricultural science that manganese plays a role of importance equal
to that of iron in the synthesis of chlorophyll, and that both of
these elements are indispensable for the formation of chlorophyll in
plants.
EXPERIMENT 617 - Nitrogen forming
bacteria
Pull up a clover or alfalfa plant and examine the roots. Notice that
along the roots there are numerous small swellings (Figure
36.) These swellings are called tubercles and if these were
crushed and examined under a powerful microscope you would find
millions of small living bacteria in them. These are the
bacteria which convert the nitrogen from the air into soluble
nitrates which are furnished to the soil and later taken up by other
plants.
EXPERIMENT 618 - To show the effect
of carbon dioxide on plant life
Procure two Mason quart jars and place about two inches of damp
earth in each. Now plant two or three peas or beans in each
jar exactly under the same conditions. Allow the jars to stand
until the beans begin to sprout. Then fill one jar with carbon
dioxide gas. The carbon dioxide gas is made by putting two spooafuls
of baking soda or sodium bicarbonate in a third empty quart jar and
adding a little vinegar or a solution of tartaric acid. After the
reaction has gone on for several moments pour the gas
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook