 The
Science Notebook
The
Science NotebookHenley's Book of Formulas, Recipes and Processes
Home Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!
Henley's Twentieth Century Book of Formulas, Recipes and Processes - Pages 76-100
[76]
ALLOYS
richer than the outside, which chills first; while with a less percentage than 72 per cent the center of the bar will be poorer and the outside richer than the average. This characteristic of silver-copper alloys is known to metallurgists as "segregation."
When nickel is added to the silver and copper, several good alloys may be formed, as the following French compositions:
I II III
Silver 33 40 20
Copper 37-42 30-40 45-55
Nickel 25-30 20-30 25-35
The whitening of alloys of silver and copper is best accomplished by annealing the alloy until it turns black on the surface. Cool in a mixture of 20 parts, by weight, of concentrated sulphuric acid to
1,000 parts of distilled water and leave therein for some time. In place of the sulphuric acid, 40 parts of potassium bisulphate may be used per 1,000 parts of liquid. Repeat the process if necessary.
Copper, Silver, and Cadmium Alloys.
Cadmium added to silver alloys gives great flexibility and ductility, without affecting the white color; these properties are valuable in the manufacture of silver-plated ware and wire. The proportions of the metals vary in these alloys. Some of the most important varieties are given below.
Silver Copper Cadmium
I 980 15 5
II 950 15 35
III 900 18 82
IV 860 20 180
V 666 25 309
VI 667 50 284
VII 500 50 450
In preparing these alloys, the great volatility of cadmium must be taken into account. It is customary to prepare first the alloy of silver and copper, and add the cadmium, which, as in the case of the alloys of silver and zinc, must be wrapped in paper. After putting it in, the mass is quickly stirred, and the alloy poured immediately into the molds. This is the surest way to prevent the volatilization of the cadmium.
Silver, Copper, Nickel, and Zinc Alloys. These alloys, from the metals contained in them, may be characterized as argentan or German silver with a certain percentage of silver. They have been used for making small coins, as in the older coins of Switzerland. Being quite hard, they have the advantage of wearing well, but soon lose their beautiful white color and take on a disagreeable shade of yellow, like poor brass. The silver contained in them can be regained only by a laborious process, which is a great drawback to their use in coinage. The composition of the Swiss fractional coins is as follows:
20 cen- 10 cen- 5 cen-
times times times
Silver 15 10 5
Copper 50 55 60
Nickel 25 25 25
Zinc 10 10 10
Mousset's Alloy. Copper, 59.06; silver, 27.56; zinc, 9.57; nickel, 3.42. This alloy is yellowish with a reddish tinge, but white on the fractured surface. It ranks next after Argent- Ruolz, which also contains sometimes certain quantities of zinc, and in this case may be classed together with the alloy just described. The following alloys can be rolled into sheet or drawn into wire:
I II III
Silver 33.3 34 40.0
Copper 41.8 42 44.6
Nickel 8.6 8 4.6
Zinc 16.3 16 10.8
Japanese (Gray) Silver. An alloy is prepared in Japan which consists of equal parts of copper and silver, and which is given a beautiful gray color by boiling in a solution of alum, to which copper sulphate and verdigris are added. The so-called "mokum," also a Japanese alloy, is prepared by placing thin plates of gold, silver, copper, and the alloy just described over each other and stretching them under the hammer. The cross sections of the thin plates obtained in this way show the colors of the different metals, which give them a peculiar striped appearance. Mokum is principally used for decorations upon gold and silver articles.
Silver-Zinc. Silver and zinc have great affinity for each other, and alloys of these two metals are therefore easily made. The required quantity of zinc, wrapped in paper, is thrown into the melted and strongly heated silver, the mass is thoroughly stirred with an iron
rod, and at once poured out into molds. Alloys of silver and zinc can be obtained which are both ductile and flexible. An alloy consisting of 2 parts of zinc and 1 of silver closely resembles silver in color, and is quite ductile. With a larger proportion of zinc the alloy becomes brittle. In preparing the alloy, a somewhat larger quantity of zinc must be taken than the
[77]
ALLOYS
finished alloy is intended to contain, as a small amount always volatilizes.
Imitation Silver Alloys. There are a number of alloys, composed of different metals, which resemble silver, and may be briefly mentioned here.
I. Warne's metal is composed of tin 10 parts, bismuth 7, and cobalt 3. It is white, fine-grained, but quite difficult to fuse.
II. Tonca's metal contains copper 5 parts, nickel 4, tin 1, lead 1, iron 1, zinc 1, antimony 1. It is hard, difficult to fuse, not very ductile, and cannot be recommended.
III. Trabuk metal contains tin 87.5, nickel 5.5, antimony 5, bismuth 5.
IV. Tourun-Leonard's metal is composed of 500 parts of tin and 64 of bell metal.
V. Silveroid is an alloy of copper, nickel, tin, zinc, and lead.
VI. Minargent. Copper, 100 parts; nickel, 70 parts; tungsten, 5 parts; aluminum, 1 part.
VII. Nickel, 23 parts; aluminum, 5 parts; copper, 5 parts; iron, 65 parts; tungsten, 4 parts.
VIII. Argasoid. Tin, 4.035; lead, 3.544; copper, 55.780; nickel, 13.406; zinc, 23.198; iron, trace.
SOLDERS:
See Solders.
STEEL ALLOYS:
See also Steel.
For Locomotive Cylinders. This mixture consists of 20 per cent steel castings, old steel, springs, etc.; 20 per cent No. 2 coke iron, and 60 per cent scrap. From this it is stated a good solid metal can be obtained, the castings being free from honeycombing, and finishing better than the ordinary cast-iron mixture, over which it has the advantage of 24 per cent greater strength. Its constituents are: Silicon, 1.51; manganese, 0.33; phosphorus, 0.65; sulphur, 0.068; combined carbon, 0.62; graphite, 2.45.
Nickel steel is composed of nickel 36 per cent, steel 64 per cent.
Tungsten steel is crucible steel with 5 to 12 per cent tungsten.
STEREOTYPE METAL.
Lead 2 parts
Tin 3 parts
Bismuth 5 parts
The melting point of this alloy is 196º F. The alloy is rather costly because of the amount of bismuth which it contains. The following mixtures are cheaper:
I II III IV
Lead 1 3 1 2
Tin 1 5 1.5 2
Bismuth 2 8 3 5
Antimony - - - 1
TIN ALLOYS:
Alloys for Dentists' Molds and Dies. I. Very hard. Tin, 16 parts; antimony, 1 part; zinc, 1 part.
II. Softer than the former. Tin, 8 parts; zinc, 1 part; antimony, 1 part.
III. Very hard. Tin, 12 parts; antimony, 2 parts; copper, 1 part.
Cadmium Alloy, about the Hardness of Zinc. Tin, 10 parts; antimony, 1 part; cadmium, 1 part.
Tin-Lead. Tin is one of those metals which is not at all susceptible to the action of acids, while lead, on the other hand, is very easily attacked by them. In such alloys, consequently, used for cooking utensils, the amount of lead must be limited, and should properly not exceed 10 or 15 per cent; but cases have been known in which the so-called tin contained a third part, by weight, of lead.
Alloys containing from 10 to 15 per cent of lead have a beautiful white color, are considerably harder than pure tin, and much cheaper. Many alloys of tin and lead are very lustrous, and are used for stage jewelry and mirrors for reflecting the light of lamps, etc. An especially brilliant alloy is called "Fahlun brilliants." It is used for stage jewelry, and consists of 29 parts of tin and 19 of lead.
It is poured into molds faceted in the same way as diamonds, and when seen by artificial light, the effect is that of diamonds. Other alloys of tin and lead are employed in the manufacture of toys. These must fill the molds well, and must also be cheap, and therefore as much as 50 per cent of lead is used. Toys can also be made from type metal, which is even cheaper than the alloys of tin and lead, but has the disadvantage of readily breaking if the articles are sharply bent. The alloys of tin and lead give very good castings, if sharp iron or brass molds are used.
Lead ............... 19 parts
Tin ................ 29 parts
This alloy is very bright and possesses a permanent sheen. It is well adapted for the making of artificial gems for stage use. It is customary in carrying out the process to start with two parts of tin and one part of lead. Tin is added until a sample drop which is allowed to fall upon an iron plate forms a mirror. The artificial gems are produced by
[78]
ALLOYS
dipping into the molten alloy pieces of glass cut to the proper shape. The tin coating of metal which adheres to the glass cools rapidly and adheres tenaciously. Outwardly these artificial gems appear rough and gray, but inwardly they are highly reflective and quite deceptive when seen in artificial light.
If the reflective surfaces be coated with red, blue, or green aniline, various colored effects can be obtained. Instead of fragile glass the gems may be produced by means of well-polished pieces of steel or bronze.
Other Tin-Lead Alloys. Percentage of lead and specific gravity.
P.O. S.G. P.O. S.G.
0 7.290 28 8.105
1 7.316 29 8.137
2 7.342 30 8.169
3 7.369 31 8.202
4 7.396 32 8.235
5 7.423 33 8.268
6 7.450 34 8.302
7 7.477 35 8.336
8 7.505 36 8.379
9 7.533 37 8.405
10 7.562 38 8.440
11 7.590 39 8.476
12 7.619 40 8.512
13 7.648 41 8.548
14 7.677 42 8.584
15 7.706 43 8.621
16 7.735 44 8.658
17 7.764 45 8.695
18 7.794 46 8.732
19 7.824 47 8.770
20 7.854 48 8.808
21 7.885 49 8.846
22 7.916 50 8.884
23 7.947 60 9.299
24 7.978 70 9.736
25 8.009 80 10.225
26 8.041 90 10.767
27 8.073 100 11.370
Tin Statuettes, Buttons, etc.
I.
Tin 4 parts
Lead 3 parts
This is a very soft solder which sharply reproduces all details.
Another easily fusible alloy but somewhat harder, is the following:
II.
Tin 8 parts
Lead 6 parts
Antimony 0.5 part
Miscellaneous Tin Alloys.
I. Alger Metal. Tin, 90 parts; antimony, 10 parts. This alloy is suitable as a protector.
II. Argentine Metal. Tin, 85.5 per cent; antimony, 14.5 per cent.
III. Ashberry metal is composed of 78 to 82 parts of tin, 16 to 20 of antimony, 2 to 3 of copper.
IV. Quen's Metal. Tin, 9 parts; lead, 1 part; antimony, 1 part; bismuth, 1 part.
Type Metal. An alloy which is to serve for type metal must be readily cast, fill out the molds sharply, and be as hard as possible. It is difficult to satisfy all these requirements, but an alloy of antimony and lead answers the purpose best. At the present day there are a great many formulas for type metal in which other metals besides lead and antimony are used, either to make the alloy more readily fusible, as in the case of additions of bismuth, or to give it greater power of resistance, the latter being of especial importance for types that are subjected to constant use. Copper and iron have been recommended for this purpose, but the fusibility of the alloys is greatly impaired by these, and the manufacture of the types is consequently more difficult than with an alloy of lead and antimony alone. In the following table some alloys suitable for casting type are given:
Lead Amtimony Copper Bismuth Zinc Tin Nickel
I 3 1 - - - - -
II 5 1 - - - - -
III 10 1 - - - - -
IV 10 2 - 1 - - -
V 70 18 2 - - 10 -
VI 60 20 - - - 20 -
VII 55 25 - - - 20 -
VIII 55 30 - - - 15 -
IX 100 30 8 2 - 20 8
X 6 - 4 - 90 - -
The French and English types contain a certain amount of tin, as shown by the following analyses:
English Types French Type
I II III
Lead 69.2 61.3 55.0 55
Antimony 19.5 18.8 22.7 30
Tin 9.1 20.2 22.1 15
Copper 1.7
Ledebur gives the composition of type metal as follows:
I II III IV
Lead 75 60 80 82
Antimony 23 25 20 14.8
Tin 22 15 3.2
WATCHMAKERS' ALLOYS:
See Watchmakers' Formulas.
WHITE METALS.
The so-called white metals are employed almost exclusively for bearings. (See Anti-friction Metals under Alloys.) In the technology of mechanics an accurate distinction is made between the different kinds of metals for bearings; and they may be classed in two groups, red brass and white metal. The red-
[79]
ALLOYS
brass bearings are characterized by great hardness and power of resistance, and are principally used for bearings of heavily loaded and rapidly revolving axles. For the axles of large and heavy flywheels, revolving at great speed, bearings of red brass are preferable to white metal, though more expensive.
In recent years many machinists have found it advantageous to substitute for the soft alloys generally in use for bearings a metal almost as hard as the axle itself. Phosphor bronze (q.V. ) is frequently employed for this purpose, as it can easily be made as hard as wrought or cast steel. In this case the metal is used in a thin layer, and serves only, as it were, to fill out the small interstices caused by wear on the axle and bearing, the latter being usually made of some rather easily fusible alloy of lead and tin. Such bearings are very durable, but expensive, and can only be used for large machines. For small machines, running gently and uniformly, white-metal bearings are preferred, and do excellent work, if the axle is not too heavily loaded. For axles which have a high rate of revolution, bearings made of quite hard metals are chosen, and with proper care which, indeed, must be given to bearings of any material they will last for a long time without needing repair.
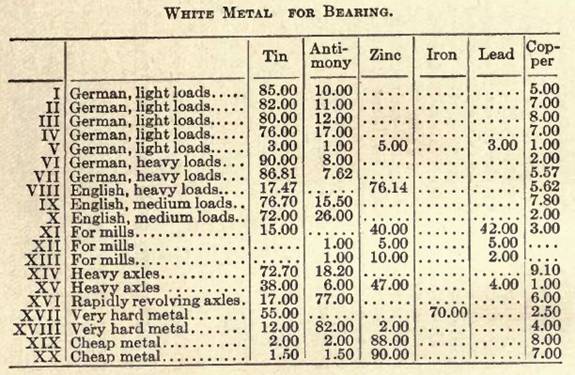
Other white bearing metals are:
XXI. Tin, 8.5; antimony, 10; copper, 5 parts.
XXII. Tin, 42; antimony, 16; lead, 42 parts.
XXIII. Tin, 72; antimony, 26; copper, 2 parts.
XXIV. Tin, 81; antimony, 12.5; copper, 6.5 parts.
White Metals Based on Copper.
I. Copper, 65 parts; arsenic, 55 parts.
II. Copper, 64 parts; arsenic, 50 parts.
III. Copper, 10 parts; zinc, 20 parts; nickel, 30 parts.
IV. Nickel, 70 parts; copper, 30 parts; zinc, 20 parts.
V. Nickel, 60 parts; copper, 30 parts; zinc, 30 parts,
VI. Copper, 8 parts; nickel, 4 parts; zinc, 4 parts.
VII. Copper, 10 parts; nickel, 5 parts; zinc, 5 parts.
VIII. Copper, 8 parts; nickel, 3 parts; zinc, 4 parts.
IX. Copper, 50 parts; nickel, 25 parts; zinc, 25 parts.
X. Copper, 55 parts; nickel, 24 parts; zinc, 21 parts.
XI. Copper, 55 parts; nickel, 24 parts; zinc, 16 parts; iron, 2 parts; tin, 3 parts.
IX, X, and XI are suitable for tableware.
XII. Copper, 67 parts, and arsenic, 53 parts.
XIII. Copper, 63 parts, and arsenic, 57 parts.
XII and XIII are bright gray, unaffected by the temperature of boiling water; they are fusible at red heat.
White Metals Based on Platinum.
I. Platinum, 1 part; copper, 4 parts; or platinum, 1 1/2 parts; copper, 3 parts.
II. Platinum, 10 parts; tin, 90 parts; or platinum, 8 parts; tin, 92 parts.
III. Platinum, 7 parts; copper, 13 parts; tin, 80 parts.
IV. Platinum, 2 parts; steel, 98 parts.
V. Platinum, 2.5 parts; steel, 97.5 parts.
IV and V are for gun metal.
Miscellaneous White-Metal Alloys.
I. For lining cross-head slides: Lead, 65 parts; antimony, 25 parts; copper, 10 parts. Some object to white metal containing lead or zinc. It has been found, however, that lead and zinc have properties of great use in these alloys.
II. Tin, 85 parts; antimony, 7 parts; copper, 7i parts.
III. Tin, 90 parts; copper, 3 parts; antimony, 7 parts.
[80]
ALUMINUM AND ITS TREATMENT
ZINC ALLOYS:
Bidery Metal. This is sometimes composed of 31 parts of zinc, 2 parts of copper, and 2 parts of lead; the whole is melted on a layer of rosin or wax to avoid oxidation. This metal is very resistive; it does not oxidize in air or moisture. It takes its name from the town of Bider, near Hyderabad (India), where it was prepared for the first time industrially for the manufacture of different utensils.
Other compositions of Indian Bidery metal (frequently imitated in England) are about as follows:

Erhardt recommends the following as being both ductile and hard:
Zinc 89 to 93
Tin 9 to 6
Lead 2 to 4
Copper 2 to 4
The tin is first melted, and the lead, zinc, and copper added successively.
Zinc-Nickel. Zinc, 90 parts; nickel, 10 parts. Used in powder form for painting and cloth printing purposes.
Platine for Dress Buttons. Copper, 43 parts; zinc, 57 parts.
UNCLASSIFIED ALLOYS:
Alloys for Drawing Colors on Steel. Alloys of various composition are successfully used for drawing colors on steel. To draw to a straw color use 2 parts of lead and 1 part of tin, and melt in an iron ladle. Hold the steel piece to be drawn in the alloy as it melts and it will turn to straw color. This mixture melts at a temperature of about 437º F. For darker yellow use 9 parts of lead to 4 parts of tin, which melts at 458º F. For purple, use 3 parts of lead to 1 part of tin, the melting temperature being 482º F. For violet, use 9 parts of lead to 2 parts of tin, which melts at 494º F. Lead without any alloy will draw steel to a dark blue. The above apply to steel only since iron requires a somewhat greater neat and is more or less uncertain in handling.
Alloy for Pattern Letters and Figures. A good alloy for casting pattern letters and figures and similar small parts of brass, iron, or plaster molds, is made of lead 80 parts, and antimony 20 parts. A better alloy will be lead 70 parts, antimony and bismuth each 15 parts. To insure perfect work the molds should be quite hot by placing them over a Bunsen burner.
Alloy for Caliper and Gage-Rod Castings. A mixture of 30 parts zinc to 70 parts aluminum gives a light and durable alloy for gage rods and caliper legs; the gage rods must be steel tipped, for the alloy is soft and wears away too rapidly for gage points.
Alloys for Small Casting Molds. Tin, 75 parts, and lead, 22 parts; or 75 parts of zinc and 25 parts of tin; or 30 parts of tin and 70 parts of lead; or 60 parts of lead and 40 parts of bismuth.
ALLOYS FOR METAL FOIL:
See Metal Foil.
ALMOND COLD CREAM:
See Cosmetics.
ALMOND LIQUEURS:
See Wines and Liquors.
ALTARS, TO CLEAN:
See Cleaning Preparations and Methods.
ALUM:
Burnt Alum.
I. Heat the alum in a porcelain dish or other suitable vessel till it liquefies, then raise and continue the heat, not allowing it to exceed 400, till aqueous vapor ceases to be disengaged, and the salt has lost 47 per cent of its weight. Reduce the residue to powder, and preserve it in a well-stoppered bottle. Cooley.
II. Heat ordinary alum (alumina alum) with constant stirring in an iron pan in which it will first melt quietly, and then commence to form blisters. Continue heating until a dry white mass of a loose character remains, which is powdered and kept in well-closed glasses.
ALUM BATH:
See Photography.
Aluminum and its Treatment
HOW TO COLOR ALUMINUM:
Blanching of Aluminum. Aluminum is one of the metals most inalterable by air; nevertheless, the objects of aluminum tarnish quickly enough without being
[81]
ALUMINUM AND ITS TREATMENT
altered. They may be restored to their mat whiteness in the following manner: Immerse the aluminum articles in a boiling bath of caustic potash; next plunge them quickly into nitric acid, rinse and let dry. It must be understood that this method is applicable only to pieces entirely of aluminum.
Decolorized Aluminum. Gray or unsightly aluminum may be restored to its white color by washing with a mixture of 30 parts of borax dissolved in 1,000 parts of water, with a few drops of ammonia added.
Mat Aluminum. In order to impart to aluminum the appearance of mat silver, plunge the article into a hot bath composed of a 10-per-cent solution of caustic soda saturated with kitchen salt. Leave it in the bath for 15 to 20 seconds, then wash and brush; put back into the bath for half a minute, wash anew and dry in sawdust.
To Blacken Aluminum.
I. The surface of the sheet to be colored is polished with very fine emery powder or finest emery cloth. After polishing pour a thin layer of olive oil over the surface and heat slowly over an alcohol flame. Large sheets must, of course, be heated in the drying oven. After a short while pour on oil again, in order to obtain absolute uniformity of the coating, and heat the plate once more. Under the action of the heat the plate turns first brown, then black, according to the degrees of heat. When the desired coloration has been attained, the plate is polished over again, after cooling, with a woolen rag or soft leather.
II.
White arsenic 1 ounce
Sulphate of iron 1 ounce
Hydrochloric acid 12 ounces
Water 12 ounces
When the arsenic and iron are dissolved by the acid add the water. The aluminum to be blackened should be well cleaned with fine emery powder and washed before immersing in the blackening solution. When the deposit of black is deep enough dry off with fine sawdust and lacquer.
Decorating Aluminum. A process for decorating aluminum, patented in Germany, prescribes that the objects be first corroded, which is usually done with caustic soda lye, or, better still, by a new method which consists in heating 3 parts of sulphuric acid with 1 part of water to 140 to 158º F., in an enameled vessel. Into this liquid dip the aluminum articles, rinsing them off clean and then drying them well. The corroded articles are now placed in a bath consisting of 1,000 parts of alcohol (90 per cent), 1.50 parts of antimony, 250 parts of chemically pure hydrochloric acid, 100 parts of manganous nitrate, and 20 parts of purified and finally elutriated graphite. In this bath, which is heated to 86-95º F., the objects are left until fumes develop around them, which takes place in a few seconds. Now they are put over a coal fire or similar arrangement until the alcohol is burned up and there is no more smoke. After they are somewhat cooled off, they are laid into cold water and worked with a brush, then rinsed with water and well dried. The pieces are now provided with a gray metallic coating, consisting mainly of antimony, manganese, and graphite. This metallic layer renders them capable of receiving a lacquer which is best prepared from 1,000 parts of alcohol (90 per cent), 50 parts of sandarac, 100 parts of shellac, and 100 parts of nigrosine (black aniline color). Then the articles are quickly but thoroughly rinsed off, dried in warmed air for a few minutes, and baked in ovens or over a moderate coal fire until they do not smoke any more and no more gloss can be seen. Finally they are rubbed with a cotton rag saturated with thin linseed-oil varnish, and the objects thus treated now appear dull black, like velvet. The covering withstands all action of the weather, so that cooking vessels coated with this varnish on the outside can be placed on the fire without injury to the coating. If the articles are engraved, the aluminum appears almost glossy white under the black layer at the engraved places. When the pieces have been provided with the gray metallic coating, colored lacquer may also be applied with the brush. In this manner paintings, etc., may be done on aluminum, while not possible on unprepared aluminum surfaces, which will not retain them.
Making Castings in Aluminum. The method adopted in preparing molds and cores for aluminum work is necessarily somewhat the same as for brass, but there are particular points which need attention to insure successful work. Both in the sand and the making of the molds there are some small differences which make considerable variation in the results, and the temperature at which the metal is poured is a consideration of some importance.
In selecting the sand, which should
[82]
ALUMINUM AND ITS TREATMENT
not have been previously used, that of a fine grain should be chosen, but it should not have any excess of aluminous matter, or it will not permit of the free escape of gases and air, this being an important matter. Besides this, the sand must be used as dry as possible consistent with its holding against the flow of the metal, and having only moderate compression in ramming.
In making the molds it is necessary to remember that aluminum has a large contraction in cooling, and also that at certain temperatures it is very weak and tears readily, while all metals shrink away from the mold when this is wholly outside the casting, but they shrink on to cores or portions of the mold partly inclosed by metal. Thus, if casting a plate or bar of metal, it will shrink away from the mold in all directions; but if casting a square frame, it shrinks away from the outside only, while it shrinks on to the central part or core. With brass, or iron, or such metals, this is not of much importance, but with some others, including aluminum, it is of great importance, because if the core or inclosed sand will not give somewhat with the contraction of the metal, torn or fractured castings will be the result. Both for outside and inside molds, and with cores used with aluminum, the sand should be compressed as little as possible, and hard ramming must in every case be avoided, particularly where the metal surrounds the sand. The molds must be very freely vented, and not only at the joint of the mold, but by using the vent wire freely through the body of the mold itself; in fact, for brass the venting would be considered excessive. With aluminum it is, however, necessary to get the air off as rapidly as possible, because the metal soon gets sluggish in the mold, and unless it runs up quickly it runs faint at the edges. The ingates should be wide and of fair area, but need careful making to prevent their drawing where they enter the casting, the method of doing this being known to most molders.
If it is considered desirable to use a specially made-up facing sand for the molds where the metal is of some thickness, the use of a little pea or bean meal will be all that is necessary. To use this, first dry as much sand as may be required and pass through a 20-mesh sieve, and to each bushel of the fine sand rub in about 4 quarts of meal, afterwards again passing through the sieve to insure regular mixing. This sand should then be damped as required, being careful that all parts are equally moist, rubbing on a board being a good way to get it tough, and in good condition, with the minimum of moisture.
The molds should not be sleeked with tools, but they may be dusted over with plumbago or steatite, smoothing with a camel's-hair brush, in cases in which a very smooth face is required on the castings. Preferably, however, the use of the brush even should be avoided.
Patterns for aluminum should be kept smooth and well varnished.
In melting the metal it is necessary to use a plumbago crucible which is clean and wnich has not been used for other metals. Clay or silica crucibles are not good for this metal, especially silica, on account of the metal absorbing silicon and becoming hard under some conditions of melting. A steady fire is necessary, and the fuel should reach only about halfway up the crucible, as it is not desirable to overheat the crucible or metal. The metal absorbs heat for some time and then fuses with some rapidity, hence the desirability of a steady heat; and as the metal should be poured when of a claret color under the film of oxide which forms on the surface, too rapid a heating is not advisable. The molding should always be well in advance of the pouring, because the metal should be used as soon as it is ready; for not only is waste caused, but the metal loses condition if kept in a molten state for long periods. The metal should be poured rapidly, but steadily, and when cast up there should not be a large head of metal left on top of the runner. In fact, it is rather a disadvantage to leave a large head, as this tends to draw rather than to feed the casting.
With properly prepared molds, and careful melting, fluxes are not required, but ground cryolite a fluoride of sodium and aluminum is sometimes used to increase the fluidity of the metal. In using this, a few ounces according to the bulk of metal to be treated is put into the molten metal before it is taken from the furnace, and well stirred in, and as soon as the reaction apparently ceases the pot is lifted and the metal at once skimmed and poured. The use of sodium in any form with aluminum is very undesirable, however, and should be avoided, and the same remark applies to tin, but there is no objection to alloying with zinc, when the metal thus produced is sold as an alloy.
Aluminum also casts very well in molds of plaster of Paris and crushed bath brick when such molds are perfectly dry
[83]
ALUMINUM AND ITS TREATMENT
and well vented, smoothness being secured by brushing over with dry steatite or plumbago. When casting in metal molds, these should be well brushed out with steatite or plumbago, and made fairly hot before pouring, as in cold molds the metal curdles and becomes sluggish, with the result that the castings run up faint.
To Increase the Toughness, Density, and Tenacity of Aluminum. For the purpose of improving aluminum, without increasing its specific gravity, the aluminum is mixed with 4 to 7 per cent of phosphorus, whereby the density, tenacity, and especially the toughness are said to be enhanced.
WORKING OF SHEET ALUMINUM:
The great secret, if there is any, in working aluminum, either pure or alloyed, consists in the proper lubricant and the shape of the tool. Another great disadvantage in the proper working of the metal is that, when a manufacturer desires to make up an article, he will procure the pure metal in order to make his samples, which, of course, is harder to work than the alloy. But the different grades of aluminum sheet which are on the market are so numerous for different classes of work that it might be advisable to consider them for a moment before passing to the method of working them.
The pure metal, to begin with, can be purchased of all degrees of hardness, from the annealed, or what is known as the "dead soft" stock, to the pure aluminum hard rolled. Then comes a harder grade of alloys, running from "dead soft" metal, which will draw up hard, to the same metal hard rolled; and, still again, another set of alloys which, perhaps, are a little harder still when hard rolled, and will, when starting with the "dead soft," spin up into a utensil which, when finished, will probably be as stiff as brass. These latter alloys are finding a large sale for replacing brass used in all classes of manufactured articles.
To start with lathe work on aluminum, probably more difficulty has been found here, especially in working pure metal, and more complaints are heard from this source than from any other. As stated before, however, these difficulties can all be readily overcome, if the proper tools and the proper lubricants are used, as automatic screw machines are now made so that they can be operated when working aluminum just as readily as when they are working brass, and in some cases more readily. To start with the question of the tool, this should be made as what is known as a "shearing tool," that is, instead of a short, stubby point, such as would be used in turning brass, the point should be lengthened out and a lot of clearance provided on the inside of the tool, so as to give the chips of the metal a good chance to free themselves and not cause a clogging around the point of the tool a similar tool, for instance, to what would be used for turning wood.
The best lubricant to be used would be coal oil or water, and plenty of it. The latter is almost as good as coal oil if enough of it is used, and with either of these lubricants and a tool properly made, there should be no difficulty whatsoever in the rapid working of aluminum, either on the lathe or on automatic screw machines.
To go from the lathe to the drawing press, the same tools here would be used in drawing up shapes of aluminum as are used for drawing up brass or other metals; the only precaution necessary in this instance being to use a proper lubricant, which in this case is a cheap grade of vaseline, or in some cases lard oil, but in the majority of instances better results will be secured by the use of vaseline. Aluminum is probably susceptible of deeper drawing with less occasion to anneal than any of the other commercial metals. It requires but one-third or one-fourth of as much annealing as brass or copper. For instance, an article which is now manufactured in brass, requiring, say, three or four operations before the article is finished, would probably have to be annealed after every operation. With aluminum, however, if the proper grade is used, it is generally possible to perform these three operations without annealing the metal at all, and at the same time to produce a finished article which, to all intents and purposes, is as stiff as an article made of sheet brass.
Too much stress cannot be laid on the fact of starting with the proper grade of metal, for either through ignorance or by not observing this point is the foundation of the majority of the complaints that aluminum "has been tried and found wanting." If, however, it should be found necessary to anneal aluminum, this can be readily accomplished by heating it in an ordinary muffle, being careful that the temperature shall not be too high - about 650 or 700º F. The best test as to when the metal has reached the proper temperature is to take a soft pine stick and draw it across the
[84]
ALUMINUM AND ITS TREATMENT
metal. If it chars the stick and leaves a black mark on the metal, it is sufficiently annealed and is in a proper condition to proceed with further operation.
Next taking up the question of spinning aluminum, success again depends particularly on starting with the proper metal. The most satisfactory speed for articles from 5 to 8 inches in diameter is about 2,600 revolutions a minute, and for larger or smaller diameters the speed should be so regulated as to give the same velocity at the circumference. Aluminum is a very easy metal to spin and no difficulty should be found at all in spinning the proper grades of sheets. Several factories that are using large quantities of aluminum now, both for spinning and stamping, are paying their men by the piece the same amount that they formerly paid on brass and tin work, and it is stated that the men working on this basis make anywhere from 10 to 20 per cent more wages by working aluminum.
After aluminum has been manufactured into the shape of an article, the next process is the finishing of it. The best polish can be obtained by first cutting down the metal with an ordinary rag buff on which use tripoli, and then finish it with a dry red rouge which comes in the lump form, or that which is known as "White Diamond Rouge." One point, however, that it is necessary to observe carefully is that both the tripoli and the rouge should be procured ground as fine as it is possible to grind them; for, if this is not done, the metal will have little fine scratches all over it, and will not appear as bright and as handsome as it otherwise would.
If it is desired to put on a frosted appearance, this can either be done by scratch brushing or sand blasting. A brass wire scratch brush, made of crimped wire of No. 32 to No. 36 B.& S. gage, with three or four rows of bristles, will probably give the best results. This work of scratch brushing can be somewhat lessened, however, if, before applying the scratch brush to the surface of the aluminum, the article is first cut down by the use of a porpoise-hide wheel and fine Connecticut sand, placing the sand between the surface of the aluminum and the wheel, so that the skin and the irregularities on the surface are removed, and then putting the article on a buffing wheel before attempting to scratch brush it. This method, however, is probably more advantageous in the treating of aluminum castings than for articles manufactured out of the sheet metal, as in the majority of cases it is simply necessary before scratch brushing to cut down the article with tripoli, and then polish it with rouge as already described, before putting on the scratch brush; in this way the brush seems to take hold quicker and better, and to produce a more uniform polish.
An effect similar to the scratch-brush finish can be got by sand blasting, and by first sand blasting and then scratch brushing the sheets, a good finish is obtained with very much less labor than by scratch brushing alone. Another very pretty frosted effect is procured by first sand blasting and then treated as hereinafter described by "dipping" and "frosting," and many variations in the finish of aluminum can be got by varying the treatment, either by cutting down with tripoli and polishing, scratch brushing, sand blasting, dipping, and frosting, and by combinations of those treatments. A very pretty mottled effect is secured on aluminum by first polishing and then scratch brushing and then holding the aluminum against a soft pine wheel, run at a high rate of speed on a lathe, and by careful manipulation, quite regular forms of a mottled appearance can be obtained.
The dipping and frosting of aluminum sheet is probably the cheapest way of producing a nice finish. First remove all grease and dirt from the article by dipping in benzine, then dip into water in order that the benzine adhering to the article may be removed, so as not to affect the strength of the solution into which it is next dipped. After they have been taken out of the water and well shaken, the articles should be plunged in a strong solution of caustic soda or caustic potash, and left there a sufficient length of time until the aluminum starts to turn black. Then they should be removed, dipped in water again, and then into a solution of concentrated nitric and sulphuric acid, composed of 24 parts of nitric acid to 1 part of sulphuric acid.
After being removed, the article should be washed thoroughly in water and dried in hot sawdust in the usual way. This finish can also be varied somewhat by making the solution of caustic soda of varying degrees of strength, or by adding a small amount of common salt to the solution.
In burnishing the metal use a bloodstone or a steel burnisher. In burnishing use a mixture of melted vaseline and coal oil, or a solution composed of 2 tablespoonfuls of ground borax dissolved in about a quart of hot water, with a few
[85]
AMALGAMS
drops of ammonia added. In engraving, which adds materially to the appearance of finished castings, book covers, picture frames, and similar articles made of sheet, probably the best lubricant to use on an engraver's tool in order to obtain a clean cut, which is bright, is naphtha or coal oil, or a mixture of coal oil and vaseline. The aphtha, however, is preferred, owing to the fact that it does not destroy the satin finish in the neighborhood of the cut, as the other lubricants are very apt to do. There is, however, as much skill required in using and making a tool in order to give a bright, clean cut as there is in the choice of the lubricant to be used. The tool should be made somewhat on the same plan as the lathe tools already outlined. That is, they should be brought to a sharp point and be "cut back" rather far, so as to give plenty of clearance.
There has been one class of work in aluminum that has been developed lately and only to a certain extent, in which there are great possibilities, and that is in drop forging the metal. Some very superior bicycle parts have been manufactured by drop forging. This can be accomplished probably more readily with aluminum than with other metals, for the reason that it is not necessary with all the alloys to work them hot; consequently, they can be worked and handled more rapidly.
ALUMINUM, TO CLEAN:
See Cleaning Preparations and Methods.
ALUMINUM ALLOYS:
See Alloys.
ALUMINUM BRONZE:
See Alloys under Bronzes.
ALUMINUM CASTINGS:
See Casting.
ALUMINUM PAPER:
See Paper.
ALUMINUM PLATING:
See Plating.
ALUMINUM POLISHES:
See Polishes.
Amalgams
See also Easily Fusible Alloys under Alloys.
The name amalgam is given to alloys of metals containing mercury. The term comes to us from the alchemists. It signifies softening, because an excess of mercury dissolves a large number of metals.
Preparation of Amalgams. Mercury forms amalgams with most metals. It unites directly and readily, either cold or hot, with potassium, sodium, barium, strontium, calcium, magnesium, zinc, cadmium, tin, antimony, lead, bismuth, silver, and gold; directly, but more difficultly, with aluminum, copper, and palladium. This combination takes place oftenest at the ordinary temperature; certain metals, however, like aluminum and antimony, combine only when heated in presence of quicksilver.
Quicksilver has no direct action on metals of high fusing points: manganese, iron, nickel, cobalt, uranium, platinum, and their congeners. Still, amalgams of these metals can be obtained of butyrous consistency, either by electrolysis of their saline solutions, employing quicksilver as the negative electrode, or by the action of an alkaline amalgam (potassium or sodium), on their concentrated and neutral saline solutions. These same refractory metals are also amalgamated superficially when immersed in the amalgam of sodium or of ammonium in presence of water.
Processes for preparing amalgams by double decomposition between an alkaline amalgam and a metallic salt, or by electrolysis of saline solutions, with employment of mercury as the negative electrode, apply a fortiori to metals capable of combining directly with the quicksilver. The latter of these methods is especially utilized for the preparation of alkaline earthy metals by electrolytic decomposition of the solutions of their salts or hydrated oxides with quicksilver as a cathode.
General Properties of Amalgams. Amalgams are liquid when the quicksilver is in great excess; solid, but readily fusible, when the alloyed metal predominates.
They have a metallic luster, and a metallic structure which renders them brittle. They even form crystallized metallic combinations of constant proportions, dissolved in an excess of quicksilver, when the excess is separated by compression in a chamois skin, or by filtration in a glass funnel of slender stem, terminating with an orifice almost capillary.
According as the fusing heat of a metal is less or greater than its combination heat with quicksilver, the amalgamation of this metal produces an elevation or a lowering of temperature. Thus potas-
[86]
AMALGAMS
sium, sodium, and cadmium, in alloy with quicksilver, disengage heat; while zinc, antimony, tin, bismuth, lead, and silver combine with mercury with absorption of heat. The amalgamation of 162 parts of quicksilver with 21 parts of lead, 12 parts of tin or of antimony, and
28.5 parts of bismuth, lowers the temperature of the mixture 79º F.
Amalgams formed with disengagement of heat are electro-negative with reference to the metals alloyed with the quicksilver. The products with absorption of heat are electro-negative with reference to the metals combined with the quicksilver; consequently, in a battery of elements of pure cadmium and amalgamated cadmium, the cadmium will be the negative pole; in case of zinc and amalgamated zinc, the zinc will be the positive pole.
Heat decomposes all amalgams, vaporizing the mercury and leaving the
metal alloys as a residue.
Water is decomposed by the amalgams of potassium and sodium, because the heat of formation of these amalgams, although considerable, is even less than the heat disengaged by potassium and sodium, on decomposing water. The alkaline amalgams may, therefore, serve as a source of nascent hydrogen in presence of water, giving rise to an action less energetic, and often more advantageous, than that of the alkaline metals alone. Thus is caused the frequent employment of sodium amalgam for hydrogenizing a large number of bodies. As a consequence of their action on water, the alkaline amalgams are changed by moist air, with production of free alkali or alkaline carbonate.
Applications of Potassium Amalgams.
I. They furnish a process for preparing potassium by the decomposition of potash by the electric current, by employing quicksilver as the cathode, and vaporizing the quicksilver of the amalgam formed by heating this in a current of dry hydrogen.
II. They can serve for the preparation of the amalgams of the metals, other than those of the alkaline group, by decomposing the salts of these metals, with formation of a salt of potash and of the amalgam of the metal corresponding to the original salt.
III. They can be employed as a source of nascent hydrogen in presence
of water for hydrogenizing many substances.
Applications of Sodium Amalgams.
These are nearly the same as those of the potassium amalgams, but the sodium amalgams are employed almost exclusively, because sodium is easier to handle than potassium, and is cheaper. These employments are the following:
I. Sodium amalgam furnishes a process for the preparation of sodium when soda is decomposed by means of the electric current, employing quicksilver as the cathode, and afterwards vaporizing the quicksilver of the amalgam formed by heating this in a current of dry hydrogen.
II. Amalgams of sodium serve for the preparation of amalgams of the other metals, particularly alkaline earthy metals and metals of high fusing points, by decomposing the salts of these metals, with formation of a salt of soda and of the amalgam of the metal corresponding to the original salt.
III. They serve for amalgamating superficially the metals of high fusing point, called "refractory," such as iron and platinum, when a well-cleaned plate of these metals is immersed in sodium amalgam in presence of water.
IV. An amalgam of 2 or 3 per cent of sodium is employed in the processes of extraction of gold by amalgamation. It has the property of rendering quicksilver more brilliant, and consequently more energetic, by acting as a deoxidant on the pellicle of oxide formed on its surface in presence of certain ores, which, by keeping it separated from the particles of gold, destroy its activity. Sodium amalgam of 3 per cent is utilized with success for the amalgamated plates employed in crushers and other apparatus for treating the ores of gold. If a few drops of this amalgam are spread on a plate of copper, of tin, or of zinc, a brilliant coating of an amalgam of tin, copper, or zinc is immediately formed.
V. Amalgams of from 2 to 8 per cent of sodium serve frequently in laboratories for reducing or hydrogenizing organic combinations, without running the risk of a partial destruction of these compounds by too intense action, as may occur by employing free sodium instead of its amalgam.
Applications of Barium Amalgams.
These can, by distillation, furnish barium. It is one of the processes for preparing this metal, which, when thus obtained, almost always retains a little sodium.
Applications of Strontium Amalgams.
These amalgams, washed and dried rapidly immediately after their preparation, and then heated to a nascent red
[87]
AMALGAMS
in a current of dry hydrogen, yield a fused mass of strontium.
Applications of Cadmium Amalgams.
Amalgams of cadmium, formed of equal weights of cadmium and quicksilver, have much power of cohesion and are quite malleable; the case is the same with an amalgam formed of 1 part of cadmium and 2 parts of quicksilver. They are used as dental cements for plugging teeth; for the same purpose an amalgam of 2 parts of quicksilver, 1 part of cadmium, and 2 parts of tin may be used.
Applications of Zinc Amalgams. The principal employment of zinc amalgams is their use as a cathode or negative electrode in the batteries of Munson, Daniels, and Lechanche. This combination is designed to render the zinc non-attackable by the exciting liquid of the battery with open circuit. The action of the mercury is to prevent the zinc from forming a large number of small voltaic elements when foreign bodies are mingled with the metal; in a word, the giving to ordinary zinc the properties of pure zinc, and consequently of causing a great saving in expense.
For amalgamating a zinc plate it is plunged for a few seconds into water in which there is one-sixteenth in volume of sulphuric acid, then rubbing with a copper-wire brush which has been dipped in the quicksilver. The mercury takes more readily on the zinc when, after the zinc has been cleaned with water sharpened with sulphuric acid, it is moistened with a solution of corrosive sublimate, which is reduced and furnishes a first very thin coat of amalgam, on which the quicksilver is immediately fixed by simple immersion without rubbing.
The zinc of a battery may be amalgamated by putting at the bottom of the compartment containing each element, a little quicksilver in such a way that the zinc touches the liquid. The amalgamation is effected under the influence of the current, but this process applies only on condition that the zinc alone touches the bottom of the vessel containing the quicksilver.
Applications of Manganese Amalgams. These may serve for the preparation
of manganese. For this purpose it is sufficient to distill in a current of pure hydrogen. The manganese remains in the form of a grayish powder.
Applications of Tin Amalgams.
I. Tinning of glass. This operation is accomplished in the following manner:
On a cast-iron table, quite horizontal, a sheet of tin of the dimensions of the glass is spread out and covered with a layer of quicksilver, 5 or 6 millimeters in thickness. The glass is made to slide on the sheet of tin in such a way as to drive off the excess of quicksilver; when the two surfaces are covered without interposition of air, weights are placed on the glass. In a few days, the glass may be removed, having been covered with an adhering pellicle of amalgam of 4 parts of tin and 1 part of quicksilver. (See also Mirrors.)
II. An amalgam consisting of 2 parts of zinc and 1 part tin may be used for covering the cushions of frictional electric machines. This amalgam is prepared by first melting the zinc and tin in a crucible and adding the quicksilver previously heated.
III. Mention has been made of the cadmium amalgam employed for plugging teeth, an amalgam of 2 parts of quicksilver, 2 parts of tin, and 1 part of cadmium. For the same purpose an amalgam of tin, silver, and gold is employed. (See also Cements, Dental.)
Applications of Copper Amalgams.
I. An amalgam of 30 per cent of copper has been employed for filling teeth. This use has been abandoned on account of the inconvenience occasioned by the great changeableness of the product.
II. The amalgam of 30 per cent of copper, designated by the name of "metallic mastic," is an excellent cement for repairing objects and utensils of porcelain. For this employment, the broken surfaces are heated to 662º F., and a little of the amalgam, previously heated to the consistency of melted wax, is applied.
III. Copper amalgam, of 30 to 45 per cent of copper, rendered plastic by heating and grinding, may serve for obtaining with slight ompression copies of delicate objects, which may, after hardening of the amalgam, be reproduced, either in wax or by galvanic process.
IV. According to Debray, when a medal, obtained with an amalgam of
45 per cent of copper, by compression in the soft state, in molds of gutta percha, is heated progressively to redness in an atmosphere of hydrogen, the quicksilver is volatilized gradually, and the particles of copper come together without fusion in such a way as to produce a faithful reproduction, formed exclusively of metallic copper, of the original medal.
V. In the metallurgy of gold the crushers are furnished with amalgamated plates of copper for retaining the gold. The preparation of these plates,
[88]
AMALGAMS
which are at least 0.128 inches in thickness, is delicate, requiring about two weeks. They are freed from greasy matter by rubbing with ashes, or, better, with a little sand and caustic soda, or if more rapid action is desired, with a cloth dipped in dilute nitric acid; they are washed with water, then with a solution of potassium cyanide, and finally brushed with a mixture of sal ammoniac and a little quicksilver, until the surface is completely amalgamated. They are finally made to absorb as much quicksilver as possible. But the plates thus treated are useful for only a few days when they are sufficiently covered with a layer of gold amalgam; in the meantime they occasion loss of time and of gold. So it is preferable to cover them artificially with a little gold amalgam, which is prepared by dissolving gold in quicksilver. Sometimes the amalgam of gold is replaced by an amalgam of silver, which is readily poured and more economical.
Another method giving better results consists in silvering copper slabs by electroplating and covering them with a layer of silver. Then it is only necessary to apply a little quicksilver, which adheres quite rapidly, so that they are ready for use almost immediately, and are quite active at the outset.
These amalgamation slabs ought to be cleaned before each operation. Potassium cyanide removes fatty matter, and sal ammoniac the oxides of the low metals.
Applications of Lead Amalgams.
These meet with an interesting employment for the autogenous soldering of lead. After the surfaces to be soldered have been well cleaned, a layer of lead amalgam is applied. It is afterwards sufficient to pass along the line of junction a soldering iron heated to redness, in order that the heat should cause the volatilization of the quicksilver, and that the lead, liberated in a state of fine division, should be melted and cause the adherence of the two surfaces. The only precaution necessary is to avoid breathing the mercurial vapor, which is quite poisonous.
Applications of Bismuth Amalgams.
The amalgam formed of 1 per cent of bismuth and 4 parts of quicksilver will cause the strong adherence of glass. It is employed with advantage in the tinning of glass globes. For this operation it is poured into a dry hot receiver, and then passed over the whole surface of the glass; it solidifies on cooling. For the purpose of economizing the bismuth, the price of which is high, the preceding amalgam is replaced by another composed of 2 parts of quicksilver, 1 part of bismuth, 1 part of lead, and 1 part of tin. The bismuth, broken into small fragments, is added to the tin and lead, previously melted in the crucible, and when the mixture of the three metals becomes fluid, the quicksilver is poured in, while stirring with an iron rod. The impurities floating on the surface are removed, and when the temperature is sufficiently lowered this amalgam is slowly poured into the vessels to be tinned, which have been previously well cleaned and slightly heated. M. Ditte recommends for the same employment, as a very strong adherent to the glass, an amalgam obtained by dissolving hot 2 parts of bismuth and
1 part of lead in a solution of 1 part of tin in 10 parts of quicksilver. By causing a quantity of this amalgam to move around the inside of a receiver, clean, dry, and slightly heated, the surface will be covered with a thin, brilliant layer, which hardens quite rapidly.
For the injection of anatomical pieces an amalgam formed of 10 parts of quicksilver, 50 parts of bismuth, 31 parts of lead, and 18 parts of tin, fusible at 77.5 and solidifiable at 60 C., is made use of; or, again, an amalgam composed of 9 parts of Darcet alloy and 1 part of quicksilver fusible at 127 1/2º F., and pasty at a still lower temperature. This last amalgam may also be used for filling carious teeth. The Darcet alloy, as known, contains 2 parts of bismuth, 1 part of lead, and 1 part of tin, and melts at 199 1/2º F. The addition of 1 part of quicksilver lowers the fusing point to 104º F.
Applications of Silver Amalgams.
I. In the silvering of mirrors by the Petitjean method, which has almost universally replaced tinning, the property of silver in readily amalgamating is taken advantage of, by substituting the glass after silvering to the action of a dilute solution of double cyanide of mercury and potassium in such a manner as to form an amalgam of white and brilliant silver adhering strongly to the glass. To facilitate the operation and utilize all the silver, while economizing the double cyanide, M. Lenoir has recommended the following: Sprinkle the glass at the time when it is covered with the mercurial solution with very fine zinc powder, which precipitates the quicksilver and regulates the amalgamation.
II. The metallurgy of silver also takes advantage of the property of this
[89]
AMALGAMS
metal in combining cold with quicksilver; this for the treatment of poor silver ores.
In the Saxon or Freiburg process for treating silver ores, recourse is had to quicksilver in the case of amalgam in amalgamating casks, in which the ore, after grinding, is shaken with disks of iron, and with mercury and water. The amalgam, collected and filtered under strong pressure, contains from 30 to 33 per cent of silver. It is distilled either in cylindrical retorts of cast iron, furnished with an exit tube immersed in the water for condensing the mercurial vapors, or on plates of iron, arranged over each other along a vertical iron stem, supported by a tripod at the bottom of a tank filled with water, and covered with an iron receiver, which is itself surrounded with ignited charcoal.
It should be remarked that the last portions of quicksilver in a silver amalgam submitted to distillation are voiaiiiized only under the action of a high and prolonged temperature.
Applications of Gold Amalgams.
I. Gilding with quicksilver. This process of gilding, much employed formerly, is now but little used. It can be applied only to metals slightly fusible and capable of amalgamation, like silver, copper, bronze, and brass. Iron can also be gilded by this method, provided it is previously covered with a coating of copper. To perform this gilding the surface is well cleaned, and the gold amalgam, consisting of 2 parts of gold and 1 part of quicksilver, prepared as mentioned before, is applied. The piece is afterwards heated to about the red, so as to volatilize the mercury. The gold remains, superficially alloyed with the metal, and forms an extremely solid layer of deadened gold, which can be afterwards polished. The volatilization should be effected under a chimney having strong draught, in order to avoid the poisonous action of the mercurial vapors.
II. The amalgamation of gold finds its principal applications in the treatment of auriferous ores. The extraction of small spangles of gold scattered in gold bearing sands is based on the ready dissolution of gold in quicksilver, and on the formation of an amalgam of solid gold by compression and filtering through a chamois skin, in a state more or less liquid. The spangles of gold are shaken with about their weight of quicksilver, collected in the cavities of sluices and mixed with a small quantity of sand. The gold is dissolved and the sand remains. The amalgam thus obtained is compressed in a chamois skin, so as to separate the excess of mercury which passes through the pores of the skin; or, yet again, it is filtered through a glass funnel having a very slender stem, with almost capillary termination. In both cases an amalgam of solid gold remains, which is submitted to the action of heat in a crucible or cast-iron retort, communicating with a bent-iron tube, of which the extremity, surrounded with a cloth immersed in water, is arranged above a receiver half full of water. The quicksilver is vaporized and condensed in the water. The gold remains in the retort.
The property of gold of combining readily with quicksilver is also used in many kinds of amalgamating apparatus for extraction and in the metallurgy of gold.
In various operations it is essential to keep the quicksilver active by preserving its limpidity. For this purpose potassium cyanide and ammonium chloride are especially employed; sometimes wood ashes, carbonate of soda, hyposulphite of soda, nitrate of potash, cupric sulphate, sea salt, and lime; the latter for precipitating the soluble sulphates proceeding from the decomposition of pyrites.
The amalgamation of gold is favored by a temperature of 38 to 45 C. (100 to 113º F.), and still more by the employment of quicksilver in the nascent state. This last property is the base of the Designol process, which consists in treating auriferous or auro-argentiferous ores, first ground with sea salt, in revolving cylinders of cast iron, with iron and mercury bichloride, in such a way that the mercury precipitated collects the gold and eventually the silver more efficaciously.
Gold Amalgam. Eight parts of gold and 1 of mercury are formed into an amalgam for plating by rendering the gold into thin plates, making it red hot, and then putting it into the mercury while the latter is also heated to ebullition. The gold immediately disappears in combination with the mercury, after which the mixture may be turned into water to cool. It is then ready for use.
Zinc Amalgam for Electric Batteries. Dissolve 2 parts of mercury in 1 part of aqua regia. This accomplished, add 5 parts of hydrochloric acid. This solution is made warm. It suffices to dip the zinc to be amalgamated into this liquid only for a few seconds.
[90]
AMALGAMS - AMBER
Amalgam for Cementing Glass, Porcelain, Etc. Take tin 2 parts, and cadmium 1 part. Fuse in an iron spoon or some vessel of the same material. When the two materials are in fusion add a little mercury, previously heated. Place all in an iron crucible and boil, agitating the mass with a pestle. This amalgam is soft and can be kneaded between the fingers. It may be employed for luting glass or porcelain vessels, as well as for filling teeth. It hardens in a short while.
Amalgam for Silvering Glass Balls. Lead, 25 parts; tin, 25 parts; bismuth, 25 parts; mercury, 25 parts; or, lead, 20 parts; tin, 20 parts; bismuth, 20 parts; mercury, 40 parts. Melt the lead and the tin, then add the bismuth; skim several times and add the mercury, stirring the composition vigorously.
(See also Mirror-Silvering).
Copper Amalgam. Copper amalgam, or so-called Viennese metal cement, crystallizes with the greatest readiness and acquires such hardness on solidifying that it can be polished like gold. The amalgam may also be worked under the hammer or between rollers; it can also be stamped, and retains its metallic luster for a long time in the air. In air containing hydrogen sulphide, however, it quickly tarnishes and turns black. A very special property of copper amalgam consists in that it becomes very soft when laid in water, and attains such pliancy that it can be employed for modeling the most delicate objects. After a few hours the amalgam congeals again into a very fine-grained, rather malleable mass. An important application of copper amalgam is that for cementing metals. All that is necessary for this purpose is to heat the metals, which must be bright, to 80-90 C. (176-194º F.), to apply the amalgam and to press the metal pieces together. They will cohere as firmly as though soldered together.
Copper amalgam may be prepared in the following manner:
Place strips of zinc in a solution of blue vitriol and agitate the solution thoroughly. The copper thus obtained in the form of a very fine powder is washed and, while still moist, treated in a mortar with a solution of mercury nitrate. The copper powder thereby amalgamates more readily with the quicksilver. Next, hot water is poured over the copper, the mortar is kept hot, and the mercury added. Knead with the pestle of the mortar until the copper, pulverulent in the beginning, has united with the mercury into a very plastic mass. The longer the kneading is continued the more uniform will be the mass. As soon as the amalgam has acquired the suitable character - for its production 3 parts of copper and 7 parts of mercury are used - the water is poured off and the amalgam still soft is given the shape in which it is to be kept.
For cementing purposes, the amalgam is rolled out into small cylinders, whose diameter is about 0.16 to 0.2 inches, with a length of a few inches. In order to produce with this amalgam impressions of castings, which are made after woodcuts, the amalgam is rolled out hot into a thin plate and pressed firmly onto the likewise heated plaster cast.
After the amalgam has hardened the thin plate of it may be reinforced by pouring on molten type metal.
Silver Amalgam. Silver amalgam can easily be made with the help of finely powdered silver. The mercury need only be heated to 250 to 300 C. (482 to 572º F.); silver powder is then sprinkled on it, and mixed with it by stirring. The vessel is heated for several minutes and then allowed to cool, the excess of mercury being removed from the granulated crystalline amalgam by pressing in a leather bag. Silver amalgam can also easily be made by dissolving silver in nitric acid, evaporating the solution till the excess of free acid is eliminated, diluting with distilled water, and adding mercury to the fluid in the proportion of 4 parts, by weight, of mercury to 1 of the silver originally used. The mercury precipitates the silver in a metallic state, and immediately forms an amalgam with it; the fluid standing above after a time contains no more silver, but consists of a solution of mercury nitrate mixed with whatever copper was contained in the dissolved silver in the form of copper nitrate. The absence of a white precipitate, if a few drops of hydrochloric acid are added to a sample of the fluid in a test tube, shows that all the silver has been eliminated from the solution and is present in the form of amalgam.
Amalgam for the Rubber of Electric Machines. Mercury, 100 parts; zinc, 50 parts; tin, 50 parts. This amalgam reduced to powder and ncorporated
with grease can be applied to the rubber of electric machines.
AMALGAM GOLD PLATING:
See Gilding under Plating.
AMBER:
Imitation Amber. Melt carefully together pine rosin, 1; lacca in tabulis, 2; white colophony, 15 parts.
[91]
AMBER CEMENT - ANILINE STAINS
AMBER CEMENT:
See Adhesives under Cements.
AMBER VARNISH:
See Varnishes.
AMBROSIA POWDER:
See Salts (Effervescent).
AMIDOL DEVELOPER:
See Photography.
AMETHYST (IMITATION):
See Gems, Artificial.
AMMON-CARBONITE:
See Explosives.
Ammonia
Household Ammonia. (See also Household Formulas.) Household ammonia is simply diluted ammonia water to which borax and soap have been added. To make it cloudy add potassium nitrate or methylated spirit. The following are good formulas:
I.
Ammonia water 16 parts
Yellow soap 64 parts
Potassium nitrate 1 part
Soft water, sufficient to make 200 parts
Shave up the soap and dissolve it in the water by heating, add the potassium nitrate and dissolve. Cool, strain, skim off any suds or bubbles, add the ammonia, mix, and bottle at once.
II.
Yellow soap 10 grains
Borax 1 drachm
Lavender water 20 minims
Stronger ammonia water 6 ounces
Water, enough to make 20 ounces
Dissolve the soap and borax in 5 ounces of boiling water; when cold add
the lavender water and ammonia, and make up to a pint with water.
III.
Methylated spirit 1 gallon
Soft water 1 gallon
Stronger ammonia water 1 gallon
IV.
Ammonia water 5 pints
Distilled water 5 pints
Soap 100 grains
Olive oil 5 drachms
Cut the soap in shavings, boil with the oil and water, cool, add the ammonia water, and bottle. For use in laundries, baths, and for general household purposes add one tablespoonful to one gallon of water.
V. The best quality:
Alcohol, 94 per cent 4 ounces
Soft water 4 gallons
Oil of rosemary 4 drachms
Oil of citronella 3 drachms
Dissolve the oils in the alcohol and add to the water. To the mixture add 4 ounces of talc (or fuller's earth will answer), mix thoroughly, strain through canvas, and to the colate add 1, 2, or 3 gallons of ammonia water, according to the strength desired, in which has been dissolved 1, 2, or 3 ounces of white curd, or soft soap.
Liquor Ainmonii Anisatus.
Oil of anise, by weight 1 part
Alcohol, by weight 24 parts
Water of ammonia, by weight 5 parts
Dissolve the oil in the alcohol and add the water of ammonia. It should be a clear, yellowish liquid.
Violet Color for Ammonia. A purple blue color may be given to ammonia water by adding an aqueous solution of litmus. The shade, when pale enough, will probably meet all views as to a violet color.
Perfumed Ammonia Water. The following are typical formulas:
I.
Stronger water of ammonia 6 ounces
Lavender water 1 ounce
Soft soap 10 grains
Water, enough to make 16 ounces
II.
Soft soap 1 ounce
Borax 2 drachms
Cologne water 1/2 ounce
Stronger water of ammonia 5 1/2 ounces
Water, enough to make 12 ounces
Rub up the soap and borax with water until dissolved, strain and add the other ingredients. The perfumes may be varied to suit the price.
AMMONIA FOR FIXING PRINTS:
See Photography.
ANGOSTURA BITTERS:
See Wines and Liquors.
ANILINE:
See Dyes.
ANILINE IN PIGMENTS, TESTS FOR:
See Pigments.
ANILINE STAINS, TO REMOVE:
See Cleaning Preparations and Methods.
[92]
ANTIDOTES FOR POISONS
ANISE CORDIAL:
See Wines and Liquors.
ANKARA:
See Butter.
ANNEALING OF STEEL, TOOLS, WIRE, AND SPRINGS:
See Steel.
ANODYNES:
See Pain Killers.
ANT DESTROYERS:
See Insecticides.
Antidotes for Poisons
POISON, SYMPTOMS AND ANTIDOTES.
When a person has taken poison the first thing to do is to compel the patient to vomit, and for that purpose give any emetic that can be most readily and quickly obtained, and which is prompt and energetic, but safe in its action. For this purpose there is, perhaps, nothing better than a large teaspoonful of ground mustard in a tumblerful of warm water, and it has the advantage of being almost always at hand. If the dry mustard is not to be had use mixed mustard from the mustard pot. Its operation may generally be facilitated by the addition of a like quantity of common table salt. If the mustard is not at hand, give two or three teaspoonfuls of powdered alum in syrup or molasses, and give freely of warm water to drink; or give 10 to 20 grains of sulphate of zinc (white vitriol), or 20 to 30 grains of ipecac, with 1 or 2 grains of tartar emetic, in a large cup of warm water, and repeat every ten minutes until three or four doses are given, unless free vomiting is sooner produced. After vomiting has taken place large draughts of warm water should be given, so that the vomiting will continue until the poisonous substances have been thoroughly evacuated, and then suitable antidotes should be given. If vomiting cannot be produced the stomach pump should be used. When it is known what particular kind of poison has been swallowed, then the proper antidote for that poison should be given; but when this cannot be ascertained, as is often the case, give freely of equal parts of calcined magnesia, pulverized charcoal, and sesquioxide of iron, in a sufficient quantity of water. This is a very harmless mixture and is likely to be of great benefit, as the ingredients, though very simple, are antidotes for the most common and active poisons. In case this mixture cannot be obtained, the stomach should be soothed and protected by the free administration of demulcent, mucilaginous, or oleaginous drinks, such as the whites of eggs, milk, mucilage of gum arabic, or slippery-elm bark, flaxseed tea, starch, wheat flour, or arrowroot mixed in water, linseed or olive oil, or melted butter or lard. Subsequently the bowels should be moved by some gentle laxative, as a tablespoonful or two of castor oil, or a teaspoonful of calcined magnesia; and pain or other evidence of inflammation must be relieved by the administration of a few drops of laudanum, and the repeated application of hot poultices, fomentations, and mustard plasters.
The following are the names of the substances that may give rise to poisoning, most commonly used, and their antidotes:
Mineral Acids Sulphuric Acid (Oil of Vitriol), Nitric Acid (Aqua Fortis), Muriatic Acid (Spirits of Salts).
Symptoms: Acid, burning taste in the mouth, acute pain in the throat, stomach, and bowels; frequent vomiting, generally bloody; mouth and lips excoriated, shriveled, white or yellow; hiccough, copious stools, more or less bloody, with great tenderness in the abdomen; difficult breathing, irregular pulse, excessive thirst, while drink increases the pain and rarely remains in the stomach; frequent but vain efforts to urinate; cold sweats, altered countenance; convulsions, generally preceding death. Nitric acid causes yellow stains; sulphuric acid, black ones. Treatment: Mix calcined magnesia in milk or water to the
consistence of cream, and give freely to drink a glassful every couple of minutes, if it can be swallowed. Common soap (hard or soft), chalk, whiting, or even mortar from the wall mixed in water may be given, until magnesia can be obtained. Promote vomiting by tickling the throat, if necessary, and when the poison is got rid of, flaxseed or slippery-elm tea, gruel, or other mild drinks. The inflammation which always follows needs good treatment to save the patient's life.
Vegetable Acids Acetic, Citric, Oxalic, Tartaric.
Symptoms: Intense burning pain of mouth, throat, and stomach; vomiting blood which is highly acid, violent purging, collapse, stupor, death.
Oxalic
acid is frequently taken in
[93]
ANTIDOTES FOR POISONS
mistake for Epsom salts, to which in shops it often bears a strong resemblance. Treatment: Give chalk or magnesia in a large quantity of water, or large draughts of limewater. If these are not at hand,
scrape the wall or ceiling, and give the scrapings mixed with water.
Prussic or Hydrocyanic Acid Laurel Water, Cyanide of Potassium, Bitter
Almond Oil, Etc.
Symptoms: In large doses almost invariably instantaneously fatal; when not immediately fatal, sudden loss of sense and control of the voluntary muscles. The odor of the poison generally noticeable on the breath. Treatment: Chlorine, in the form of chlorine water, in doses of from 1 to 4 fluidrachms, diluted. Weak solution of chloride lime of soda; water of ammonia (spirits of hartshorn), largely diluted, may be given, and the vapor of it cautiously inhaled. Cold affusion, and chloroform in half to teaspoonful doses in glycerine or mucilage, repeated every few minutes, until the symptoms are ameliorated. Artificial respiration.
Aconite Monkshood, Wolfsbane.
Symptoms: Numbness and tingling in the mouth and throat, and afterwards in other portions of the body, with sore throat, pain over the stomach, and vomiting; dimness of vision, dizziness, great prostration, loss of sensibility, and delirium. Treatment: An emetic and then brandy in tablespoonful doses, in ice water, every half hour; spirits of ammonia in half-teaspoonful doses in like manner; the cold douche over the head and chest, warmth to the extremities, etc.
Alkalis and Their Salts Concentrated Lye, Wood-ash Lye, Caustic Potash, Ammonia, Hartshorn.
Symptoms: Caustic, acrid taste, excessive heat in the throat, stomach, and intestines; vomiting of bloody matter, cold sweats, hiccough, purging of bloody stools. Treatment: The common vegetable acids. Common vinegar, being always at hand, is most frequently used. The fixed oils, as castor, flaxseed, almond, and olive oils form soaps with the alkalis and thus also destroy their caustic effect. They should be given in large quantity.
Antimony and Its Preparations Tartar Emetic, Antimonial Wine, Kerme's Mineral.
Symptoms: Faintness and nausea, soon followed by painful and continued vomiting, severe diarrhea, constriction and burning sensation in the throat, cramps, or spasmodic twitchings, with symptoms of nervous derangement, and great prostration of strength, often terminating in death. Treatment: If vomiting has not been produced, it should be ffected by tickling the fauces, and administering copious draughts of warm water. Astringent infusions, such as of gall, oak bark, Peruvian bark, act as antidotes, and should be given promptly. Powdered yellow bark may be used until the infusion is prepared, or very strong green tea should be given. To stop the vomiting, should it continue, blister over the stomach by applying a cloth wet with strong spirits of hartshorn, and then sprinkle on one-eighth to one-fourth of a grain of morphia.
Arsenic and Its Preparations Ratsbane, Fowler's Solution, Etc.
Symptoms: Generally within an hour pain and heat are felt in the stomach, soon followed by vomiting, with a burning dryness of the throat and great thirst; the matters vomited are generally colored either green yellow, or brown, and are sometimes bloody. Diarrhea or dysentery ensues, while the pulse becomes small and rapid, yet irregular. Breathing much oppressed; difficulty in vomiting may occur, while cramps, convulsions, or even paralysis often precede death, which sometimes takes place within five or six hours after arsenic has been taken. Treatment: Give a prompt emetic, and then hydrate of peroxide of iron (recently prepared) in tablespoonful doses every 10 or 15 minutes until the urgent symptoms are relieved. In the absence of this, or while it is being prepared, give large draughts of new milk and raw eggs, limewater and oil, melted butter, magnesia in a large quantity of water, or even if nothing else is at hand, flour and water, always 5> however, giving an emetic the first thing, or causing vomiting by tickling the throat with a feather, etc. The inflammation of the stomach which follows must be treated by blisters, hot fomentations, mucilaginous drinks, and the like.
Belladonna, or Deadly Nightshade.
Symptoms: Dryness of the mouth and throat, great thirst, difficulty of swallowing, nausea, dimness, confusion or loss of vision, great enlargement of the pupils, dizziness, delirium, and coma. Treatment: There is no known antidote. Give a prompt emetic and then reliance must be placed on continual stimulation with brandy, whisky, etc., and to necessary artificial respiration. Opium and its preparations, as morphia, laudanum, etc., are thought by some to
[94]
ANTIDOTES FOR POISONS
counteract the effect of belladonna, and may be given in small and repeated doses, as also strong black coffee and green tea.
Blue Vitriol, or Blue Stone.
See Copper.
Cantharides (Spanish or Blistering Fly) and Modern Potato Bug.
Symptoms: Sickening odor of the breath, sour taste, with burning heat in the throat, stomach, and bowels; frequent vomiting, often bloody; copious bloody stools, great pain in the stomach, with burning sensation in the bladder and difficulty to urinate followed with terrible convulsions, delirium, and death. Treatment: Excite vomiting by drinking plentifully of sweet oil or other wholesome oils, sugar and water, milk, or slippery-elm tea; give injections of castor oil and starch, or warm milk. The inflammatory symptoms which generally follow must be treated by a physician. Camphorated oil or camphorated spirits should be rubbed over the bowels, stomach, and thighs.
Caustic Potash.
See Alkalis under this title.
Cobalt, or Fly Powder.
Symptoms: Heat and pain in the throat and stomach, violent retching and vomiting, cold and clammy skin, small and feeble pulse, hurried and difficult breathing, diarrhea, etc. Treatment: An emetic, followed by the free administration of milk, eggs, wheat flour and water, and mucilaginous drinks.
Copper Blue Vitriol, Verdigris or Pickles or Food Cooked in Copper Vessels.
Symptoms: General inflammation of the alimentary canal, suppression of urine; hiccough, a disagreeable metallic taste, vomiting, violent colic, excessive thirst, sense of tightness of the throat, anxiety; faintness, giddiness, and cramps and convulsions generally precede death. Treatment: Large doses of simple syrup as warm as can be swallowed, until the stomach rejects the amount it contains. The whites of eggs and large quantities of milk. Hydrated peroxide of iron.
Creosote - Carbolic Acid.
Symptoms: Burning pain, acrid, pungent taste, thirst, vomiting, purging, etc. Treatment: An emetic and the free administration of albumen, as the whites of eggs, or, in the absence of these, milk, or flour and water.
Corrosive Sublimate.
See Mercury under this title.
Deadly Nightshade.
See Belladonna under this title.
Foxglove, or Digitalis.
Symptoms: Loss of strength, feeble, fluttering pulse, faintness, nausea and vomiting and stupor; cold perspiration, dilated pupils, sighing, irregular breathing, and sometimes convulsions. Treatment: After vomiting, give brandy and ammonia in frequently repeated doses, apply warmth to the extremities, and if necessary resort to artificial respiration.
Gases - Carbonic Acid, Chlorine, Cyanogen, Hydrosulphuric Acid, Etc.
Symptoms: Great drowsiness, difficult respiration, features swollen, face blue as in strangulation. Treatment: Artificial respiration, cold douche, friction with stimulating substances to the surface of the body. Inhalation of steam containing preparations of ammonia. Cupping from nape of neck. Internal use of chloroform.
Hellebore, or Indian Poke.
Symptoms: Violent vomiting and purging, bloody stools, great anxiety, tremors, vertigo, fainting, sinking of the pulse, cold sweats, and convulsions. Treatment: Excite speedy vomiting by large draughts of warm water, molasses and water, tickling the throat with the finger
or a feather, and emetics; give oily and mucilaginous drinks, oily purgatives, and clysters, acids, strong coffee, camphor, and opium.
Hemlock (Conium).
Symptoms: Dryness of the throat, tremors, dizziness, difficulty of swallowing, prostration, and faintness, limbs powerless or paralyzed,
pupils dilated, pulse rapid and feeble; insensibility and convulsions sometimes precede death. Treatment: Empty the stomach and give brandy in tablespoonful doses, with half teaspoonful of spirits of ammonia, frequently repeated, and if much pain and vomiting, give bromide of ammonium in 5-grain doses every half hour. Artificial respiration may be required.
Henbane, or Hyoscyamus.
Symptoms: Muscular twitching, inability to articulate plainly, dimness of vision and stupor; later, vomiting and purging, small intermittent pulse, convulsive movement of the extremities, and coma. Treatment: Similar to opium poisoning, which see.
Iodine.
Symptoms: Burning pain in throat, lacerating pain in the stomach, fruitless effort to vomit, excessive tenderness of the epigastrium. Treatment:
[95]
ANTIDOTES FOR POISONS
Free emesis, prompt administration of starch, wheat flour, or arrowroot, beaten up in water.
Lead Acetate of Lead, Sugar of Lead, Dry White Lead, Red Lead, Litharge, or Pickles, Wine, or Vinegar Sweetened by Lead.
Symptoms: When taken in large doses, a sweet but astringent metallic taste exists, with constriction in the throat, pain in the region of the stomach, painful, obstinate, and frequently bloody vomitings, hiccough, convulsions or spasms, and death. When taken in small but long-continued doses it produces colic, called painters' colic; great pain, obstinate constipation, and in extreme cases paralytic symptoms, especially wrist-drop, with a blue line along the edge of the gums. Treatment: To counteract the poison give alum in water 1 1/2 ounce to a quart; or, better still, Epsom salts or Glauber's salts, an ounce of either in a quart of water; or dilute sulphuric acid, a teaspoonful to a quart of water. If a large quantity of sugar of lead has been recently taken, empty the stomach by an emetic of sulphate of zinc (1 drachm in a quart of water), giving one-fourth to commence, and repeating smaller doses until free vomiting is produced; castor oil should be given to clear the bowels and injections of oil and starch freely administered. If the body is cold use the warm bath.
Meadow Saffron.
See Belladonna.
Laudanum.
See Opium.
Lobelia Indian Poke.
Symptoms: Excessive vomiting and purging, pains in the bowels, contraction of the pupils, delirium, coma, and convulsions. Treat-
ment: Mustard over the stomach, and brandy and ammonia.
Mercury Corrosive Sublimate (bug poisons frequently contain this poison), Red Precipitate, Chinese or English Vermilion.
Symptoms: Acrid, metallic taste in the mouth, immediate constriction and burning in the throat, with anxiety and tearing pains in both stomach and bowels, sickness, and vomiting of various-colored fluids, and sometimes bloody and profuse diarrhea, with difficulty and pain in urinating; pulse quick, small, and hard; faint sensations, great debility, difficult breathing, cramps, cold sweats, syncope, and convulsions. Treatment: If vomiting does not already exist, emetics must be given immediately white of eggs in continuous large doses, and infusion of catechu afterwards, sweet milk, mixtures of flour and water in successive cupfuls, and to check excessive salivation put a half ounce of chlorate of potash in a tumbler of water, and use freely as a gargle, and swallow a tablespoonful every hour or two.
Morphine.
See Opium.
Nitrate of Silver (Lunar Caustic).
Symptoms: Intense pain and vomiting, and purging of blood, mucus, and shreds of mucous membranes; and if these stand they become dark. Treatment: Give freely of a solution of common salt in water, which decomposes the poison, and afterwards flaxseed or slippery-elm-bark tea, and after a while a dose of castor oil.
Opium and All Its Compounds Morphine, Laudanum, Paregoric, Etc.
Symptoms: Giddiness, drowsiness, increasing to stupor, and insensibility; pulse usually, at first, quick and irregular, and breathing hurried, and afterwards pulse slow and feeble, and respiration slow and noisy; the pupils are contracted and the eyes and face congested, and later, as death approaches, the extremities become cold, the surface is covered with cold, clammy perspiration, and the sphincters relax. The effects of opium and its preparations, in poisonous doses, appear in from a half to two hours from its administration. Treatment: Empty the stomach immediately with an emetic or with the stomach pump. Then give very strong coffee without milk; put mustard plasters on the wrists and ankles; douche the head and chest with cold water, and if the patient is cold and sinking, give brandy, or whisky and ammonia. Belladonna is thought by many to counteract the poisonous effects of opium, and may be given in doses of half to a teaspoonful of the tincture, or 2 grains of the extract, every 20 minutes, until some effect is observed in causing the pupils to expand. Use warmth and friction, and if possible prevent sleep for some hours, for which purpose the patient should be walked about between two persons. Finally, as a last resort, use artificial respiration, persistence in which will sometimes be rewarded with success in apparently hopeless cases. Electricity should also be tried.
Cooley advises as follows: Vomiting must be induced as soon as ossible, by means of a strong emetic and tickling the fauces. If this does not succeed, the stomach pump should be applied. The emetic may consist of a half drachm of sulphate of zinc dissolved in a half pint of warm water, of which one-third should
[96]
ANTIDOTES FOR POISONS
be taken at once, and the remainder at the rate of a wineglassful every 5 or 10 minutes, until vomiting commences. When there is much drowsiness or stupor 1 or 2 fluidrachms of tincture of capsicum will be found a useful addition; or one of the formulas for emetic draughts may be taken instead. Infusion of galls, cinchona, or oak bark should be freely administered before the emetic, and water soured with vinegar and lemon juice, after the stomach has been well cleared out. To rouse the system spirit and water or strong coffee may be given. To keep the sufferer awake, rough friction should be applied to the skin, an upright posture preserved, and walking exercise enforced, if necessary.
When this is ineffectual cold water may be dashed over the chest, head, and spine, or mild shocks of electricity may be had recourse to. To allow the sufferer to sleep is to abandon him to destruction. Bleeding may be subsequently necessary in plethoric habits, or in threatened congestion. The costiveness that accompanies convalescence may be best met by aromatic aperients; and the general tone of the habit restored by stimulating tonics and the shower bath. The smallest fatal dose of opium in the case of an adult within our recollection was 4 grains. Children are much more susceptible to the action of opium than of other medicines, and hence the dose of it for them must be diminished considerably below that indicated by the common method of calculation depending on the age.
Oxalic Acid.
See Acids.
Phosphorus Found in Lucifer Matches and Some Rat Poisons.
Symptoms: Symptoms of irritant poisoning; pain in the stomach and bowels; vomiting, diarrhea; tenderness and tension of the abdomen. Treatment: An emetic is to be promptly given; copious draughts containing magnesia in suspension; mucilaginous drinks. General treatment for inflammatory symptoms.
Poisonous Mushrooms.
Symptoms: Nausea, heat and pains in the stomach and bowels; vomiting and purging, thirst, convulsions, and faintings; pulse small and frequent, dilated pupil and stupor, cold sweats and death. Treatment: The stomach and bowels are to be cleared by an emetic of ground mustard
or sulphate of zinc, followed by frequent doses of Glauber's or of Epsom salts, and large stimulating clysters. After the poison is evacuated, either may be given with small quantities of brandy and water. But if inflammatory symptoms manifest themselves such stimuli should be avoided, and these symptoms appropriately treated. A hypodermic injection of 1/62 grain of atropine is the latest discovered antidote.
Potash.
See AlkalI.
Prussic or Hydrocyanic Acid.
See Acids.
Poison Ivy.
Symptoms: Contact with, and with many persons the near approach to, the vine gives rise to violent erysipelatous inflammation, especially of the face and hands, attended with itching, redness, burning, and swelling, with watery blisters. Treatment: Give saline laxatives, and apply weak sugar of lead and laudanum, or limewater and sweet oil, or bathe the parts freely with spirits of niter. Anointing with oil will prevent poisoning from it.
Saltpeter (Nitrate of Potash).
Symptoms: Only poisonous in large quantities, and then causes nausea, painful vomiting, purging, convulsions, faintness, feeble pulse, cold feet and hands, with tearing pains in stomach and bowels. Treatment: Treat as is directed for arsenic, for there is no antidote known, and emptying the stomach and bowels with mild drinks must be relied on.
Savine.
Symptoms: Sharp pains in the bowels, hot skin, rapid pulse, violent
vomiting and sometimes purging, with great prostration. Treatment: Mustard and hot fomentations over the stomach and bowels and ice allowed in the stomach only until the inflammation ceases. If prostration comes on, food and stimulants must be given by injection.
Stramonium, Thorn Apple, or Jamestown Weed.
Symptoms: Vertigo, headache, perversion of vision, slight delirium, sense of suffocation, disposition to sleep, bowels relaxed, and all secretions augmented. Treatment: Same as for belladonna.
Snake Bites, Cure for. The Inspector of Police in the Bengal Government reports that of 939 cases in which ammonia was freely administered, 207 victims have recovered, and in the cured instances the remedy was not administered till about 3 1/2 hours after the attack; on the average of the fatal cases the corresponding duration of time was 4 1/2 hours.
Strychnine or Nux Vomica. The characteristic symptom is the special influence exerted upon the nervous system,
[97]
ANTIDOTES FOR POISONS
which is manifested by a general contraction of all the muscles of the body, with rigidity of the spinal column. A profound calm soon succeeds, which is followed by a new tetanic seizure, longer than the first, during which the respiration is suspended. These symptoms then cease, the breathing becomes easy, and there is stupor, followed by another general contraction. In fatal cases these attacks are renewed, at intervals, with increasing violence, until death ensues. One phenomenon which is found only in poisonings by substances containing strychnine is that touching any part of the body, or even threatening to do so, instantly produces the tetanic spasm. Antidote: The stomach should be immediately cleared by means of an emetic, tickling the fauces, etc. To counteract the asphyxia from tetanus, etc., artificial respiration should be practiced with diligence and care. "If the poison has been applied externally, we ought immediately to cauterize the part, and apply a ligature tightly above the wound. If the poison has been swallowed for some time we should give a purgative clyster, and administer draughts containing sulphuric ether or oil of turpentine, which in most cases produce a salutary effect. Lastly, injections of chlorine and decoction of tannin are of value."
According to Ch. Gunther the greatest reliance may be placed on full doses of opium, assisted by venesection, in cases of poisoning by strychnia or nux vomica. His plan is to administer this drug in the form of solution or mixture, in combination with a saline aperient.
Another treatment is to give, if obtainable, 1 ounce or more of bone charcoal mixed with water, and follow with an active emetic; then to give chloroform in teaspoonful doses, in flour and water or glycerine, every few minutes while the spasms last, and afterwards brandy and stimulants, and warmth of the extremities if necessary. Recoveries have followed the free and prompt administration of oils or melted butter or lard. In all cases empty the stomach if possible.
Sulphate of Zinc White Vitriol.
See Zinc.
Tin Chloride of Tin, Solution of Tin (used by dyers), Oxide of Tin, or Putty Powder.
Symptoms: Vomiting, pains in the stomach, anxiety, restlessness, frequent pulse, delirium, etc. Treatment: Empty the stomach, and give whites of eggs in water, milk in large quantities, or flour beaten up in water, with magnesia or chalk.
Tartar Emetic.
See Antimony.
Tobacco.
Symptoms: Vertigo, stupor, fainting, nausea, vomiting, sudden nervous debility, cold sweat, tremors, and at times fatal prostration. Treatment: After the stomach is empty apply mustard to the abdomen and to the extremities, and give strong coffee, with brandy and other stimulants, with warmth to the extremities.
Zinc Oxide of Zinc, Sulphate of Zinc, White Vitriol, Acetate of Zinc.
Symptoms: Violent vomiting, astringent taste, burning pain in the stomach, pale countenance, cold extremities, dull eyes, fluttering pulse. Death seldom ensues, in consequence of the emetic effect. Treatment: The vomiting may be relieved by copious draughts of warm water. Carbonate of soda, administered in solution, will decompose the sulphate of zinc. Milk and albumen will also act as antidotes. General principles to be observed in the subsequent treatment.
Woorara.
Symptoms: When taken into the stomach it is inert; when absorbed through a wound it causes sudden stupor and insensibility, frothing at the mouth, and speedy death. Treatment: Suck the wound immediately, or cut it out and tie a cord around the limb between the wound and the heart. Apply iodine, or iodide of potassium, and give it internally, and try artificial respiration.
ANTIFERMENTS.
The following are tried and useful formulas:
I. Sulphite (not sulphate) of lime, in fine powder, 1 part; marble dust, ground oyster shells, or chalk, 7 parts; mix, and pack tight, so as to exclude the air.
II. Sulphite (not sulphate) of potassa, 1 part; new black-mustard seed (ground in a pepper mill), 7 parts; mix, and pack so as to exclude air and moisture perfectly. Dose (of either), 1/2 ounce to 1 1/2 ounces per hogshead.
III. Mustard seed, 14 pounds; cloves and capsicum, of each, 1 1/4 pounds; mix, and grind them to powder in a pepper mill. Dose, 1/4 to 1/2 pound per hogshead.
A portion of any one of these compounds added to cider, or the like, soon allays fermentation, when excessive, or when it has been renewed. The first formula is preferred when there is a tendency to acidity. The second and third may be advantageously used for wine and beer, as
[98]
ANTISEPTICS
well as for cider. The third compound greatly improves the flavor and the apparent strength of the liquor, and also improves its keeping qualities.
Anchovy Preparations
Extemporaneous Anchovy Sauce.
Anchovies, chopped small 3 or 4
Butter 3 ounces
Water 2 ounces
Vinegar 1 ounce
Flour 1 ounce
Mix, place over the fire, and stir until the mixture thickens. Then rub through a coarse sieve.
Essence of Anchovies. Remove the bones from 1 pound of anchovies, reduce the remaining portions of the fish to a pulp in a Wedgewood mortar, and pass through a clean hair or brass sieve. Boil the bones and other portions which will not pass through the sieve in 1 pint of water for 15 minutes, and strain. To the strained liquor add 2 1/2 ounces of salt and 2 1/2 ounces of flour, and the pulped anchovies. Let the whole simmer over the fire for three or four minutes; remove from the fire, and when the mixture has cooled a little add 4 ounces of strong vinegar. The product (nearly 3 pounds) may be then bottled, and the corks tied over with bladder, and either waxed or capsuled.
Anchovy Paste.
Anchovies 7 pounds
Water 9 pints
Salt 1 pound
Flour 1 pound
Capsicum 1/4 ounce
Grated lemon peel 1
Mushroom catsup 4 ounces
Anchovy Butter.
Anchovies, boned and beaten to a paste 1 part
Butter 2 parts
Spice enough
ANTIFOULING COMPOSITIONS:
See Paints.
ANTIFREEZING SOLUTION:
See Freezing Preventives.
ANTIFRICTION METAL:
See Alloys, under Phosphor Bronze and Antifriction Metals.
ANTIQUES, TO PRESERVE.
The best process for the preservation of antique metallic articles consists in a retransformation of the metallic oxides into metal by the electrolytic method. For this purpose a zinc strip is wound around the article and the latter is laid in a soda-lye solution of 5 per cent, or suspended as the negative pole of a small battery in a potassium cyanide solution of 2 per cent. Where this method does not seem practicable it is advisable to edulcorate the objects in running water, in which operation fragile or easily destroyed articles may be protected by winding with gauze; next, they should be carefully dried, first in the air, then with moderate heat, and finally protected from further destruction by immersion in melted paraffine. A dry place is re
quired for storing the articles, since paraffine is not perfectly impermeable to water in the shape of steam.
ANTIRUST COMPOSITIONS:
See Rust Preventives.
Antiseptics
Antiseptic Powders.
I.
Borax 3 ounces
Dried alum 3 ounces
Thymol 22 grains
Eucalyptol 20 drops
Menthol 1 1/2 grains
Phenol 15 grains
Oil of gaultheria 4 drops
Carmine to give a pink tint.
II.
Parts by weight
Alum, powdered 50
Borax, powdered 50
Carbolic acid, crystals 5
Oil of eucalyptus 5
Oil of wintergreen 5
Menthol 5
Thymol 5
III.
Boracic acid 10 ounces
Sodium biborate 4 ounces
Alum 1 ounce
Zinc sulphocarbolate 1 ounce
Thymic acid 1 drachm.
Mix thoroughly. For an antiseptic wash dissolve 1 or 2 drachms in a quart of warm water.
IV.
Ektogan is a new dusting powder which is a mixture of zinc hydroxide and dioxide. It is equivalent to about 8 per cent of active oxygen. It is a yellowish-white odorless and tasteless powder, insoluble in water. It is used externally in wounds and in skin diseases as a moist dressing mixed with citric, tartaric, or
[99]
ANTISEPTICS
tannic acid, which causes the liberation of oxygen. With iodides it liberates iodine. It is stated to be strongly antiseptic; it is used in the form of a powder, a gauze, and a plaster.
Antiseptic Pencils.
I.
Tannin q.s
Alcohol, q.s 1 part
Ether, q.s 3 parts
Make into a mass, using as an excipient the alcohol and ether previously mixed. Roll into pencils of the desired length and thickness. Then coat with collodion, roll in pure silver leaf, and
finally coat with the following solution of gelatine and set aside to dry:
Gelatine 1 drachm
Water 1 pint
Dissolve by the aid of a gentle heat. When wanted for use, shave away a portion of the covering, dip the pencil into tepid water and apply.
II. Pencils for stopping bleeding are prepared by mixing:
Parts by weight
Purified alum 480
Borax 24
Oxide zinc 2 1/2
Thymol 8
Formalin 4
Melting carefully in a water bath, adding some perfume, and forming mixture into pencils or cones.
A very convenient way to form into pencils where no mold need be made is to take a small glass tube, roll a piece of oil paper around the tube, remove the glass tube, crimp the paper tube thus formed on one end and stand it on end or in a bottle, and pour the melted solution in it and leave until cool, then remove the paper.
Antiseptic Paste (Poison) for Organic Specimens.
(a)
Wheat flour 16 ounces
Beat to a batter with cold water 16 fluidounces.
Then pour into boiling water 32 fluidounces
(b)
Pulverized gum arabic 2 ounces
Dissolve in boiling water 4 fluidounces
(c)
Pulverized alum 2 ounces
Dissolve in boiling water 4 fluidounces
(d)
Acetate of lead 2 ounces
Dissolve in boiling water 4 fluidounces
(e)
Corrosive sublimate 10 grains
Mix (a) and (b) while hot and continue to simmer; meanwhile stir in (c) and mix thoroughly; then add (d). Stir briskly, and pour in the dry corrosive sublimate. This paste is very poisonous. It is used for anatomical work and for pasting organic tissue, labels on skeletons, etc.
Mouth Antiseptics.
I. Thymic acid, 25 centigrams (3 1/4 grains): benzoic acid, 3 grams (45 grains); essence of peppermint, 75 centigrams (10 minims); tincture of eucalyptus, 15 grams (4 1/2 drachms) ; alcohol, 100 grams (3 ounces).
Put sufficient in a glass of water to render latter milky.
II. Tannin, 12 grams (3 drachms); menthol, 8 grams (2 drachms); thymol,
1 gram (15 grains); tincture benzoin, 6 grams (90 minims); alcohol, 100 grams (3 ounces). Ten drops in a half-glassful of tepid water.
See also Dentifrices for Mouth Washes.
Antiseptic Paste. Difficulty is often experienced in applying an antiseptic dressing to moist surfaces, such as the lips after operation for harelip. A paste for this purpose is described by its originator, Socin. The composition is: Zinc oxide, 50 parts; zinc chloride, 5 parts; distilled water, 50 parts. The paste is applied to the wound, previously dried by means of a brush or spatula, allowed to dry on, and to remain in place five or six days. It may then be removed and a fresh application made.
Potassium bicarbonate 32.0 grams
Sodium benzoate 32.0 grams
Sodium borate 8.0 grams
Thymol 0.2 gram
Eucalyptol 2.0 c. cent.
Oil of peppermint 0.2 c. cent.
Oil of wintergreen 0.4 c. cent.
Tincture of cudbear 15.0 c. cent.
Alcohol 60.0 c. cent.
Glycerine 250.0 c. cent.
Water, enough to make 1,000.0 c. centimeters
Dissolve the salts in 650 cubic centimeters of water, and the thymol, eucalyptol, and oils in the alcohol. Mix the alcoholic solution with the glycerine and add the aqueous liquid, then the tincture of cudbear, and lastly enough water to make 1,000 cubic centimeters. Allow to stand a few days, then filter, adding a little magnesium carbonate to the filter, if necessary, to get a brilliant filtrate.
This is from the Formulary of the Bournemouth Pharmaceutical Association, as reported in the Canadian Pharmaceutical Association:
[100]
ANTISEPTICS
Alkaline Glycerine of Thymol.
Sodium bicarbonate 100 grains
Sodium biborate 200 grains
Sodium benzoate 80 grains
Sodium salicylate 40 grains
Menthol 2 grains
Pumilio pine oil 4 minims
Wintergreen oil 2 minims
Thymol 4 grains
Eucalyptol 12 minims
Compound Solution of Thymol.
A
Benzoic acid 64 grains
Borax 64 grains
Boric acid 128 grains
Distilled water 6 ounces
Dissolve.
B
Thymol 20 grains
Menthol 6 grains
Eucalyptol 4 minims
Oil of wintergreen 4 minims
Oil of peppermint 2 minims
Oil of thyme 1 minim
Alcohol (90 per cent) 3 ounces
Dissolve.
Mix solutions A and B, make up to 20 fluidounces with distilled water, and filter.
Oil of Cinnamon as an Antiseptic. Oil of cinnamon in a 9-per-cent emulsion, when used upon the hands, completely sterilizes them. A 7-to 8-per-cent emulsion is equal to a 1-per-cent solution of corrosive sublimate and is certainly far more agreeable to use. Oil of thyme in an 11-per-cent solution is equal to a 7-per-cent solution of cinnamon oil.
Green Coloring for Antiseptic Solutions. The safest coloring substance for use in a preparation intended either for internal administration or for application to the skin is the coloring matter of leaves, chlorophyll. A tincture of spinach or of grass made by macerating 2 ounces of the freshly cut leaves in a pint of alcohol for five days will be found to give good results. If the pure coloring substance is wanted the solvent should be evaporated off.
Antiseptic Bromine Solution.
Bromine 1 ounce
Sodium chloride 8 ounces
Water 8 pints
Dissolve the sodium chloride in the water and add the bromine. This solution is to be diluted, when applied to broken skin surfaces, 1 part with 15 parts of water.
Substitute for Rubber Gloves. Murphy has found that a 4-, 6-, or 8-per-cent solution of gutta-percha in benzine, when applied to the hands of the surgeon or the skin of the patient, will seal these surfaces with an insoluble, impervious, and practically imperceptible coating - a coating that will not allow the secretions of the skin to escape, and will not admit secretions, blood, or pus into the crevices of the skin. At the same time it does not impair the sense of touch nor the pliability of the skin. A similar solution in acetone also meets most of the requirements.
Murphy's routine method of hand preparation is as follows: First, five to seven minutes' scrubbing with spirits of green soap and running hot water; second, three minutes' washing with alcohol; third, when the hands are thoroughly dried, the gutta-percha solution is poured over the hands and forearms, care being taken to fill in around and beneath the nails. The hands must be kept exposed to the air with the fingers separated until thoroughly dry. The coating is very thin and can be recognized only by its glazed appearance. It will resist soap and water, but is easily removed by washing in benzine. The hands can be washed in bichloride or any of the antiseptic solutions without interfering with the coating or affecting the skin. If the operations be many, or prolonged, the coating wears away from the tips of the fingers, but is easily renewed. For the remaining portion of the hands one application is sufficient for a whole morning's work.
The 4-per-cent solution of rubber wears better on the tips of the fingers, in handling instruments, sponges, and tissues than the acetone solution.
For the abdomen the acetone solution has the advantage, and it dries in three to four seconds after its application, while the benzine solution takes from three to four and a half minutes to make a dry, firm coating.
The preparation of the patient's skin consists in five minutes' scrubbing with spirits of green soap, washing with ether, followed by alcohol. The surface is then swabbed over thoroughly with the benzine or acetone solution.
The gutta-percha solution is prepared by dissolving the pure gutta-percha chips in sterile benzine or acetone. These solutions do not stand boiling, as this impairs the adhesiveness and elasticity of
the coating.
ANTISEPTICS FOR CAGED BIRDS:
See Veterinary Formulas.