 The
Science Notebook
The
Science NotebookHenley's Book of Formulas, Recipes and Processes
Home Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!
Henley's Twentieth Century Book of Formulas, Recipes and Processes - Pages
[276]
DYES
enable the operator to form shades almost without number.
The manufacture of the egg dyes as carried on in the factory consists in a mechanical mixing of basic coal-tar dyestuffs, also some direct coloring benzidine dyestuffs, with dextrin in the ratio of about 1 part of aniline dye to 8 parts of dextrin; under certain circumstances, according to the concentrated state of the dyes, the reducing quantity of the dextrin may be greatly increased. As reducing agents for these colors insoluble substances may also be employed. A part also of the egg dyes are treated with the neutral sulphate; for instance, light brilliant green, because of its rubbing off, is made with dextrin and Glauber's salt in the proportion of 1:3:3.
For the dyeing of eggs such color mixtures are preferably employed as contain along with the dye proper a fixing agent (dextrin) as well as a medium for the superficial mordanting of the eggshell. The colors will then be very brilliant.
Here are some recipes:
|
Color |
Dyestuff |
Parts by Weight |
Cit. Acid |
Dextrin |
|
Blue |
Marine blue B.N. |
3.5 |
35.0 |
60.0 |
|
Brown |
Vesuvin S |
30.0 |
37.5 |
30.0 |
|
Green |
Brilliant green O |
13.5 |
18.0 |
67.5 |
|
Orange |
Orange II |
9.0 |
18.0 |
75.0 |
|
Red |
Diamond fuschine I |
3.5 |
18.0 |
75.0 |
|
Pink |
Eosin A |
4.5 |
|
90.0 |
|
Violet |
Methyl violet 6B |
3.6 |
18.0 |
75.0 |
|
Yellow |
Naphthol yellow S |
13.5 |
36.0 |
67.5 |
Very little of these mixtures suffices for dyeing five eggs. The coloring matter is dissolved in 600 parts by weight of boiling water, while the eggs to be dyed are boiled hard, whereupon they are placed in the dye solution until they seem sufficiently colored. The dyes should be put up in waxed paper.
Fast Stamping Color. Rub up separately, 20 parts of cupric sulphate and 20 parts of anilic hydrochlorate, then mix carefully together, after adding 10 parts of dextrin. The mixture is next ground with 5 parts of glycerine and sufficient water until a thick, uniform, paste-like mass results, adapted for use by means of stencil and bristle-brush. Aniline black is formed thereby in and upon the fiber, which is not destroyed by boiling.
New Mordanting Process. The ordinary method of mordanting wool with
a bichromate and a reducing agent always makes the fiber more or less tender, and Amend proposed to substitute the use of a solution of chromic acid containing 1 to 2 per cent of the weight of the wool, at a temperature not exceeding 148º F., and to treat it afterwards with a solution of sodium bisulphite. According to a recent French patent, better results are obtained with neutral or slightly basic chromium sulphocyanide. This salt, if neutral or only slightly basic, will mordant wool at 148º F. The double sulphocyanide of chromium and ammonium, got by dissolving chromic oxide in ammonium sulphocyanide, can also be used. Nevertheless, in order to precipitate chromium chromate on the fiber, it is advisable to have a soluble chromate and a nitrate present, as well as a soluble copper salt and a free acid. One example of the process is as follows: Make the bath with 2 to 3 per cent of ammoniochromium sulphocyanide, one-half of 1 per cent sodium bichromate, one-third of 1 per cent sodium nitrite, one-third of 1 per cent sulphate of copper, and 1.5 per cent sulphuric acid percentages based on the weight of the wool. Enter cold and slowly heat to about 140 to 150º F. Then work for half an hour, lift and rinse. The bath does not exhaust and can be reinforced and used again.
Process for Dyeing in Khaki Colors. Bichromate of potash or of soda, chloride of manganese, and a solution of acetate of soda or formiate of soda (15º Bé.) are dissolved successively in equal quantities.
The solution thus composed of these three salts is afterwards diluted at will, according to the color desired, constituting a range from a dark brown to a light olive green shade. The proportions of the three salts may be increased or diminished, in order to obtain shades more or less bister.
Cotton freed from its impurities by the usual methods, then fulled as ordinarily, is immersed in the bath. After a period, varying according to the results desired, the cotton, threads, or fabrics of cotton, are washed thoroughly and plunged, still wet, into an alkaline solution, of which the concentration ought never to be less than 14º Bé. This degree of concentration is necessary to take hold of the fiber when the cotton comes in contact with the alkaline bath, and by the contraction which takes place the oxides of chrome and of manganese remain fixed in the fibers.
This second operation is followed by washing in plenty of water, and then the cotton is dried in the open air. If the color is judged to be too pale, the threads or fabrics are immersed again in the initial bath, left the necessary time for obtaining the desired shade, and then
[277]
DYES
washed, but without passing them through an alkaline bath. This process furnishes a series of khaki colors, solid to light, to fulling and to chlorine.
LAKES:
Scarlet Lake. In a vat holding 120 gallons provided with good agitating apparatus, dissolve 8 pounds potash alum in 10 gallons hot water and add 50 gallons cold water. Prepare a solution of 2 pounds ammonia soda and add slowly to the alum solution, stirring all the time. In a second vessel dissolve 5 pounds of brilliant scarlet aniline, by first making it into a paste with cold water and afterwards pouring boiling water over it; now let out steam into the vat until a temperature of 150º to 165º F. is obtained. Next dissolve 10 pounds barium chloride in 10 gallons hot water in a separate vessel, add this very slowly, stir at least 3 hours, keeping up temperature to the same figures. Fill up vat with cold water and leave the preparation for the night. Next morning the liquor (which should be of a bright red color) is drawn off, and cold water again added. Wash by decantation 3 times, filter, press gently, and make into pulp.
It is very important to precipitate the aluminum cold, and heat up before adding the dyestuff. The chemicals used for precipitating must be added very slowly and while constantly stirring. The quantity used for the three washings is required each time to be double the quantity originally used.
I. Madder Lakes. Prepare from the root 1 pound best madder, alum water (1 pound alum with 1 1/2 gallons of water), saturated solution of carbonate of potash (3/4 pound carbonate of potash to 1/2 gallon of water).
The madder root is inclosed in a linen bag of fine texture, and bruised with a pestle in a large mortar with 2 gallons of water (free from lime) added in small quantities at a time, until all the coloring matter is extracted. Make this liquor boil, and gradually pour into the boiling water solution. Add the carbonate of potash solution gradually, stirring all the time. Let the mixture stand for 12 hours and drop and dry as required.
II. Garancine Process. This is the method usually employed in preference to that from the root. Garancine is prepared by steeping madder root in sulphate of soda and washing.
Garancine 2 pounds
Alum (dissolved in a little water) 2 pounds
Chloride of tin 1/2 ounce
Sufficient carbonate of potash or soda to precipitate the alum.
Boil the garancine in 4 gallons of pure water; add the alum, and continue boiling from 1 to 2 hours. Allow the product to partially settle and filter through flannel before cooling. Add to the filtrate the chloride of tin, and sufficient of the potash or soda solution to precipitate the alum; filter through flannel and wash well. The first filtrate may be used for lake of an inferior quality, and the garancine originally employed may also be treated as above, when a lake slightly inferior to the first may be obtained.
Maroon Lake. Take of a mixture made of:
2/3 Sapan wood |
1/3 Lima wood | 56 parts
Soda crystals 42 parts
Alum 56 parts
Extract the color from the woods as for rose pink, and next boil the soda and alum together and add to the woods solution cold. This must be washed clean before adding to the wood liquor.
Carnation Lake.
Water 42 gallons
Cochineal 12 pounds
Salts of tartar 1 1/2 pounds
Potash alum 3/4 pound
Nitrous acid, nitromuriate of tin 44 pounds
Muriatic acid, nitromuriate of tin 60 pounds
Pure block tin, nitromuriate of tin 22 pounds
Should give specific gravity 1.310.
Boil the water with close steam, taking care that no iron touches it; add the cochineal and boil for not more than five minutes; then turn off the steam and add salts of tartar and afterwards carefully add the alum. If it should not rise, put on steam until it does, pass through a
120-mesh sieve into a settling vat, and let it stand for 48 hours (not for precipitation). Add gradually nitromuriate of tin until the test on blotting paper (given below) shows that the separation is complete. Draw off clear water after it has settled, and filter. To test, rub a little of the paste on blotting paper, then dry on steam chest or on the hand, and if on bending it cracks, too much tin has been used.
To Test the Color to See if it is Precipitating. Put a drop of color on white blotting paper, and if the color spreads, it is not precipitating. If there is a color-
[278]
DYES
less ring around the spot of color it shows that precipitation is taking place; if the white ring is too strong, too much has been used.
BLACK LAKES FOR WALL-PAPER
MANUFACTURE:
Bluish-Black Lake. Boil well 220 parts of Domingo logwood in 1,000 parts of water to which 2 parts of ammonia soda have been added; to the boiling logwood add next 25 parts of green vitriol and then 3.5 parts of sodium bichromate. The precipitated logwood lake is washed out well twice and then filtered.
Black Lake AI. Logwood extract, Sanford, 120 parts; green vitriol, 30 parts; acetic acid, 7º Bé., 10 parts; sodium bichromate, 16 parts; powdered alum, 20 parts. The logwood extract is first dissolved in boiling water and brought to 25º Bé. by the addition of cold water.
Then the remaining ingredients are added in rotation, the salts in substance, finely powdered, with constant stirring. After the precipitation, wash twice and filter.
Aniline Black Lake. In the precipitating vat filled with 200 parts of cold water enter with constant stirring in the order mentioned the following solutions kept in readiness: Forty parts of alum dissolved in 800 parts of water; 10 parts of calcined soda dissolved in 100 parts of water; 30 parts of azo black dissolved in 1,500 parts of water; 0.6 parts of "brilliant green" dissolved in 100 parts of water; 0.24 parts of new fuchsine dissolved in 60 parts of water; 65 parts of barium chloride dissolved in 1,250 parts of water. Allow to settle for 24 hours, wash the lake three times and filter it.
Carmine Lake for Wall Paper and Colored Papers. Ammonia soda (98 per cent), 57.5 parts by weight; spirits (96 per cent), 40 parts by weight; corallin (dark), 10 parts by weight; corallin (pale), 5 parts by weight; spirit of sal ammoniac (16º Bé.), 8 parts by weight; sodium phosphate, 30 parts by weight; stannic chloride, 5 parts by weight; barium chloride, 75 parts by weight. Dissolve the corallin in the spirit, and filter the solution carefully into eight bottles, each containing 1 part of the above quantity of spirit of sal ammoniac, and let stand. The soda should meanwhile be dissolved in hot water and the solution run into the stirring vat, in which there is cold water to the height of 17 inches. Add the sodium phosphate, which has been dissolved in a copper vessel, then the corallin solution, and next the stannic chloride diluted with 3 pailfuls of cold water. Lastly the barium chloride solution is added. The day previous barium chloride is dissolved in a cask in as little boiling water as possible, and the receptacle is filled entirely with cold water. On the day following, allow the same to run in slowly during a period of three fourths of an hour, stir till evening, allow to settle for 2 days, draw off and filter.
English Pink.
Quercitron bark 200 parts
Lime 10 parts
Alum 10 parts
Terra alba 300 parts
Whiting 200 parts
Sugar of lead 7 parts
Put the bark into a tub, slake lime in another tub, and add the clear limewater to wash the bark; repeat this 3 times, letting the bark stand in each water 24 hours. Run liquor into the tub below and add the terra alba and whiting; wash well in the top tub and run into liquor below through a hair sieve, stirring well.
Dissolve the sugar of lead in warm water and pour gently into the tub, stirring all the time; then dissolve the alum and run in while stirring; press slightly, drop, and dry as required.
Dutch Pink.
I.
Quercitron bark 200 parts
Lime 20 parts
Alum 20 parts
Whiting 100 parts
Terra alba 200 parts
White sugar of lead 10 parts
II.
Quercitron bark 300 parts
Lime 10 parts
Alum 10 parts
Terra alba 400 parts
Whiting 100 parts
Sugar of lead 7 parts
Put the bark into a tub with cold water, slake 28 pounds of lime, and add the limewater to the bark. (This draws all the color out of the wood.) Dissolve alum in water and run it into bark liquor. The alum solution must be just warm. Dissolve sugar of lead and add it to above, and afterwards add the terra alba and whiting. The product should now be in a pulp, and must be dropped and dried as required.
Rose Pink.
I. Light.
Sapan wood 100 parts
Lima 100 parts
Paris white 200 parts
Alum 210 parts
[279]
DYES
II. Deep.
Sapan wood 300 parts
Lima 300 parts
Terra alba 400 parts
Paris white 120 parts
Lime 12 parts
Alum 200 parts
III.
Sapan wood 200 parts
Alum 104 parts
Whiting 124 parts
Boil the woods together in 4 waters and let the products stand until cold; wash in the whiting and terra alba through a hair sieve, and afterwards run in the alum. If a deep color is required slake 12 pounds lime and run it in at the last through a hair sieve. Let the alum be just warm or it will show in the pink.
DYES, COLORS, ETC., FOR TEXTILE GOODS:
Aniline Black. This black is produced by carefully oxidizing aniline hydrochloride. The exact stage of oxidation must be carefully regulated or the product will be a different body (quinone). There are several suitable oxidizing agents, such as chromic acid, potassic bichromate, ferrocyanide of potassium, etc., but one of the easiest to manipulate is potassic chlorate, which by reacting on copper sulphate produces potassic sulphate and copper chlorate. This is easily decomposed, its solution giving off gases at 60º F. which consist essentially of chloride anhydrate. But one of the most useful agents for the production of aniline black is yanadate of ammonia, 1 part of which will do the work of 4,000 parts of copper. Many other salts besides copper may be used for producing aniline black, but the following method is one of the best to follow in making this dye:
Aniline hydrochloride 40 parts
Potassic chlorate 20 parts
Copper sulphate 40 parts
Chloride of ammonia (sal ammoniac) 16 parts
Warm water at 60º F 500 parts
After warming a few minutes the mass froths up. The vapor should not be inhaled. Then set aside, and if the mass is not totally black in a few hours, again heat to 60º F., and expose to the air for a few days, and finally wash away all the soluble salts and the black is fit for use.
Aniline Black Substitutes.
I. Make a solution of
Aniline (fluid measure) 30 parts
Toluidine (by weight). 10 parts
Pure hydrochloric acid, B. P. (fluid measure) 60 parts
Soluble gum arabic (fluid measure) 60 parts
Dissolve the toluidine in the aniline and add the acid, and finally the mucilage.
II. Mix together at gentle heat:
Starch paste 13 quarts
Potassic chlorate 350 scruples
Sulphate of copper 300 scruples
Sal ammoniac 300 scruples
Aniline hydrochloride 800 scruples
Add 5 per cent of alizarine oil, and then steep it for 2 hours in the dye bath of red liquor of 2 1/2º Tw. Dye in a bath made up of 1/2 ounce of rose bengal and 1 1/2 ounces of red liquor to every 70
ounces of cotton fabric dyed, first entering the fabric at 112º F., and raising it to 140º F., working for 1 hour, or until the desirable shade is obtained; then rinse and dry.
Blush Pink on Cotton Textile. Rose bengal or fast pink will give this shade. The mordant to use is a 5 per cent solution of stannate of soda and another 5 per cent solution of alum.
Dissolve in a vessel (a) 8 1/2 parts of chloride of copper in 30 parts of water, and then add 10 parts chloride of sodium and 9 1/2 parts liquid ammonia.
In a second vessel dissolve (b) 30 parts aniline hydrochlorate in 20 parts of water, and add 20 parts of a solution of gum arabic prepared by dissolving 1 part of gum in 2 parts of water.
Finally mix 1 part of a with 4 parts of b; expose the mixture to the air for a few days to develop from a greenish to a black color. Dilute for use, or else dry the thick compound to a powder.
If new liquor is used as the mordant, mix 1 part of this with 4 parts of water, and after working the fabric for 1 to 2 hours in the cold liquor, wring or squeeze it out and dry; before working it in the dye liquor, thoroughly wet the fabric by rinsing it in hot water at a spring boil; then cool by washing in the dye bath until the shade desired is attained, and again rinse and dry.
The red liquor or acetate of aluminum may be made by dissolving 13 ounces of alum in 69 ounces of water and mixing this with a solution made by dissolving 7 1/2 ounces of acetate of lime, also dissolved in 69 ounces of water. Stir well, allow it to settle, and filter or decanter
[280]
DYEING
off the clear fluid for use, and use this mixture 2 1/2º Tw.
The fabric is first put into the stannate of soda mordant for a few minutes, then wrung out and put into the alum mordant for about the same time; then it is again wrung out and entered in the dye bath at 120º F. and dyed to shade desired, and afterwards rinsed in cold water and dried.
The dye bath is made of 1/4 ounce of rose bengal per gallon of water. If fast pink is the dye used, the mordant used would be Turkey red oil and red liquor. Use 8 ounces of Turkey red oil per gallon of water. Put the fabric into this, then wring out the textile and work in red liquor of 7º Tw. for about 2 hours, then wring out and dye in a separate bath made up of cosine, or fast pink, in water in which a little alum has been dissolved.
To Dye Woolen Yarns, etc., Various Shades of Magenta. To prepare the dye bath dissolve 1 pound of roseine in 15 gallons of water. For a concentrated solution use only 10 gallons of water, while if a very much concentrated color is needed, dissolve the dye in methylated spirit of wine, and dilute this spirituous tincture with an equal quantity of water.
No mordant is required in using this color in dyeing woolen goods. The dyeing operation consists simply in putting the goods into the dye bath at 190º F. and working them therein until the desired shade is obtained, then rinsing in cold water and drying.
If the water used in preparing the dye is at all alkaline, make use of the acid roseine dissolved in water in which a little sulphuric acid has been mixed, and work, gradually raising to the boiling point, and keep up the temperature for 30 minutes, or according to the shade desired. Put about 20 per cent sulphate of soda into the dye oath.
Maroon Dye for Woolens. To prepare the dye bath, dissolve about 1 pound of maroon dye in boiling water, with or without the addition of methylated spirit of wine. For dark shades dissolve in boiling water, only slightly acidulated with hydrochloric acid, and filter before use. No mordant is required with this dye when dyeing wool, but for the bright shade a little curd soap may be dissolved in the dye bath before proceeding to dye the wool, while for the dark shade it is best to put in a little acetate of soda. To use the dye, first dye in a weak bath and gradually strengthen it until the desired shade is obtained, at the same time gradually increasing the temperature until just below the boiling point.
To Dye Woolens with Blue de Lyons. Dissolve 8 ounces of blue dye in 1 gallon of methylated spirit, which has been slightly soured with sulphuric acid, and boil the solution over a water bath until it is perfectly clear. To prepare the dye bath, add more or less of the spirituous tincture to a 10- or 15-gallon dye bath of water, which has been slightly soured with sulphuric acid.
Rich Orange on Woolen. Dissolve 1 pound of phosphine in 15 gallons of boiling water, and stir the fluid until the acid has dissolved. No mordant is required to dye wool. First work the goods about in a weak solution, and finally in one of full strength, to which a little acetate of soda has been added. Keep up the temperature to just below the boiling point while working the goods in the dye bath.
DYEING SILK OR COTTON FABRICS WITH ANILINE DYES:
Aniline Blue on Cotton. Prepare a dye bath by dissolving 1 pound of aniline blue (soluble in spirit) in 10 gallons of water, and set it aside to settle. Meanwhile prepare a mordant while boiling 35 ounces of sumac (or 5 ounces tannic acid in 30 gallons of water) and then dissolve therein 17 ounces of curd soap. Boil up and filter. Put the cotton goods in the hot liquid and let them remain therein for 12 hours. Then wring them out and make up a dye bath of 2 1/2º Tw. with red liquor. Add dye color according to the shade desired.
Put in the goods and work them until the color is correct, keeping the temperature at the boiling point.
To Dye Silk a Delicate Greenish Yellow. Dissolved ounces of citronine in 1 gallon of methylated spirit and keep the solution hot over a water bath until perfectly clear.
To prepare silk fabrics, wash them in a weak soap liquor that has been just sweetened (i.e., its alkalinity turned to a slight sourness) with a little sulphuric acid. Work the goods until dyed to shade, and then rinse them in cold water that has been slightly acidulated with acetic, tartaric, or citric acid.
To Dye Cotton Dark Brown. Prepare a mordant bath of 10 pounds of catechu, 2 pounds of logwood extract, and 1/4 pound magenta (roseine), and bring to a boil; work the goods therein for 3 hours at that temperature; then put
[281]
DYEING
into a fresh dye bath made up of 3 pounds of bichromate of potash and 2 pounds of sal soda, and dye to shade. These proportions are for a dye bath to dye 100 pounds of cotton goods at a time.
To Dye Silk Peacock Blue. Make up a dye bath by putting 1 pint of sulphuric acid at 170º Tw., and 10 ounces of methylin blue crystal dye liquor of 120 to 160º Tw., with a dye bath that will hold 80 pounds of goods. Put in the silk at 130º F., and raise to 140º F., and work up to shade required.
To Dye Felt Goods. Owing to this material being composed of animal and vegetable fiber it is not an easy matter always to produce evenness of shade. The best process to insure success is to steep well the felt in an acid bath of from 6º to 12º Bé., and then wash away all traces of acid. Some dyers make the fulling stork the medium of conveying the dye, while others partially dye before fulling, or else dye after that process.
The fulling stock for 72 ounces of beaver consists of a mixture of
Black lead or plumbago 16 ounces
Venetian red 48 ounces
Indigo extract (fluid) 5 ounces
Ordinary Drab.
Common plumbago 12 ounces
Best plumbago 12 ounces
Archil extract (fluid) 15 ounces
Indigo extract 10 ounces
Mix into fluid paste with water and add sulphuric acid at 30º Tw. For the dye liquor make a boiling-hot solution of the aniline dye and allow it to cool; then put into an earthenware vessel holding water and heat to 83º F., and add sufficient dye liquor to give the quantity of felt the desired shade. First moisten well the felted matter (or the hair, if dyed before felting) with water, and then work it about in the above dye bath at 140º F. To deepen the shade, add more dye liquor, lifting out the material to be dyed before adding the fresh dye liquor, so that it can be well stirred up and thoroughly mixed with the exhausted bath.
Brown Shades. Bismarck brown will give good results, particularly if the dyed goods are afterwards steeped or passed through a weak solution (pale straw color) of bichromate of potash. This will give a substantial look to the color. Any of the aniline colors suitable for cotton or wool, or those suited for mixed cotton and wool goods may be used.
Blue. Use either China blue, dense ferry blue, or serge blue, first making the material acid before dyeing.
Green. Use brilliant green and have the material neutral, i.e, neither acid nor alkali; or else steep in a bath of sumac before dyeing.
Plum Color. Use maroon (neutral or acid) and work in an acid bath or else sumac.
Black. Use negrosin in an acid bath, or else mordant in two salts and dye slightly acid.
Soluble Blue, Ball Blue, etc. A soluble blue has for many years been readily obtainable in commerce which is similar in appearance to Prussian blue, but, unlike the latter, is freely soluble in water.
This blue is said to be potassium ferri-ferrocyanide.
To prepare instead of buying it ready made, gradually add to a boiling solution of potassium ferricyanide (red prussiate of potash) an equivalent quantity of hot solution of ferrous sulphate, boiling for 2 hours and washing the precipitate on a filter until the washings assume a dark-blue color. The moist precipitate can at once be dissolved by the further addition of a sufficient quantity of water. About 64 parts of the iron salt is necessary to convert 100 parts of the potassium salt into the blue compound.
If the blue is to be sent out in the liquid form, it is desirable that the solution should be a perfect one. To attain that end the water employed should be free from mineral substances, and it is best to filter the solution through several thicknesses of fine cotton cloth before bottling; or if made in large quantities this method may be modified by allowing it to stand some days to settle, when the top portion can be siphoned off for use, the bottom only requiring filtration.
The ball blue sold for laundry use consists of ultramarine. Balls or tablets of this substance are formed by mixing it with glucose or glucose and dextrin, and pressing into shape. When glucose alone is used, the product has a tendency to become soft on keeping, which tendency may be counteracted by a proper proportion of dextrin. Bicarbonate of sodium is added as a filler to cheapen the product, the quantity used and the quality of the ultramarine employed being both regulated by the price at which the product is to sell.
New Production of Indigo. Forty parts of a freshly prepared ammonium
sulphide solution containing 10 per cent
[282]
DYEING EGGS
of hydrogen sulphide are made to flow quickly and with constant stirring into a heated solution of 20 parts of isatine anilide in 60 parts of alcohol. With spontaneous heating and temporary green and blue coloration, an immediate separation of indigo in small crystalline needles of a faint copper luster takes place. Boil for a short time, whereupon the indigo is filtered off, rewashed with alcohol, and dried.
To Dye Feathers. A prerequisite to the dyeing of feathers appears to be softening them, which is sometimes accomplished by soaking them in warm water, and sometimes an alkali, such as ammonium or sodium carbonate, is added. This latter method would apparently be preferable on account of the removal of any greasy matter that may be present.
When so prepared the feathers may be dyed by immersion in any dye liquor. An old-time recipe for black is immersion in a bath of ferric nitrate suitably diluted with water, and then in an infusion of equal parts of logwood and quercitron. Doubtless an aniline dye would prove equally efficient and would be less troublesome to use.
After dyeing, feathers are dipped in an emulsion formed by agitating any bland fixed oil with water containing a little potassium carbonate, and are then dried by gently swinging them in warm air. This operation gives the gloss.
Curling where required is effected by slightly warming the feathers before a fire, and then stroking with a blunt metallic edge, as the back of a knife. A certain amount of manual dexterity is necessary to carry the whole process to a successful ending.
DYES FOR FOOD:
See Foods.
DYES FOR LEATHER:
See Leather.
DYE STAINS, THEIR REMOVAL FROM THE SKIN:
See Cleaning Preparations and Methods.
DYNAMITE:
See Explosives.
EARTHENWARE:
See Ceramics.
EAU DE QUININE:
See Hair Preparations.
EBONY:
See Wood.
EBONY LACQUER:
See Lacquers.
ECZEMA DUSTING POWDER FOR CHILDREN.
Starch, French chalk, lycopodium, of each, 40 parts; bismuth subnitrate, 2 parts; salicylic acid, 2 parts; menthol, 1 part. Apply freely to the affected parts.
Eggs
The age of eggs may be approximately judged by taking advantage of the fact that as they grow old their density decreases through evaporation of moisture. According to Siebel, a new-laid egg placed in a vessel of brine made in the proportion of 2 ounces of salt to 1 pint of water, will at once sink to the bottom. An egg 1 day old will sink below the surface, but not to the bottom, while one 3 days old will swim just immersed in the liquid. If more than 3 days old the egg will float on the surface, the amount of shell exposed increasing with age; and if 2 weeks old, only a little of the shell will dip in the liquid.
The New York State Experiment Station studied the changes in the specific gravity of the eggs on keeping and found that on an average fresh eggs had a specific gravity of 1.090; after they were 10 days old, of 1.072; after 20 days, of 1.053; and after 30 days, of 1.035. The test was not continued further. The changes in specific gravity correspond to the changes in water content. When eggs arc kept they continually lose water by evaporation through the pores in the shell. After 10 days the average loss was found to be 1.60 per cent of the total water present in the egg when perfectly fresh; after 20 days, 3.16 per cent; and after 30 days, 5 per cent. The average temperature of the room where the eggs were kept was 63.8º F. The evaporation was found to increase somewhat with increased temperature. None of the eggs used in the 30-day test spoiled.
Fresh eggs are preserved in a number of ways which may, for convenience, be grouped under two general classes: (1) Use of low temperature, i.e, cold storage; and (2) excluding the air by coating, covering, or immersing the eggs, some material or solution being used which may or may not be a germicide. The two methods are often combined. The
[283]
EGGS
first method owes its value to the fact that microorganisms, like larger forms of plant life, will not grow below a certain temperature, the necessary degree of cold varying with the species. So far as experiment shows, it is impossible to kill these minute plants, popularly called "bacteria" or "germs," by any degree of cold; and so, very low temperature is unnecessary for preserving eggs, even if it were not undesirable for other reasons, such as injury by freezing and increased cost. According to a report of the Canadian commission of agriculture and dairying:
Eggs are sometimes removed from the shells and stored in bulk, usually on a commercial scale, in cans containing about 50 pounds each. The temperature recommended is about 30º F., or a little below freezing, and it is said they will keep any desired length of time. They must be used soon after they have been removed from storage and have been thawed.
Water glass or soluble glass is the popular name for potassium silicate, or sodium silicate, the commercial article often being a mixture of the two. The commercial water glass is used for preserving eggs, as it is much cheaper than the chemically pure article which is required for many scientific purposes. Water glass is commonly sold in two forms, a syrup-thick liquid of about the consistency of molasses, and a powder. The thick syrup, the form perhaps most usually seen, is sometimes sold wholesale as low as 1 3/4 cents per pound in carboy lots. The retail price varies, though 10 cents per pound, according to the North Dakota Experiment Station, seems to be the price commonly asked. According to the results obtained at this station a solution of the desired strength for preserving eggs may be made by dissolving 1 part of the syrup-thick water glass in 10 parts, by measure, of water. If the water-glass powder is used, less is required for a given quantity of water. Much of the water glass offered for sale is very alkaline. Such material should not be used, as the eggs preserved in it will not keep well. Only pure water should be used in making the solution, and it is best to boil it and cool it before mixing with the water glass.
The solution should be carefully poured over the eggs packed in a suitable vessel, which must be clean and sweet, and if wooden kegs or barrels are used they should be thoroughly scalded before packing the eggs in them. The packed eggs should be stored in a cool place. If they are placed where it is too warm, silicate deposits on the shell and the eggs do not keep well. The North Dakota Experiment Station found it best not to wash the eggs before packing, as this removes the natural mucilaginous coating on the outside of the shell. The station states that 1 gallon of the solution is sufficient for 50 dozen eggs if they are properly packed.
It is, perhaps, too much to expect that eggs packed in any way will be just as satisfactory for table use as the fresh article. The opinion seems to be, however, that those preserved with water glass are superior to most of those preserved otherwise. The shells of eggs preserved in water glass are apt to crack in boiling. It is stated that this may be prevented by puncturing the blunt end of the egg with a pin before putting it into the water.
To Discover the Age of Eggs. The most reliable method of arriving at the age of hens' eggs is that by specific gravity. Make a solution of cooking salt (sodium chloride) in rain or distilled water, of about one part of salt to two parts of water, and in this place the eggs to be tested. A perfectly fresh egg (of from 1 to 36 hours old) will sink completely, lying horizontally on the bottom of the vessel; when from two to three days old, the egg also sinks, but not to the, bottom, remaining just below the surface of the water, with a slight tendency of the large end to rise. In eggs of four or five days old this tendency of the large end to rise becomes more marked, and it increases from day to day, until at the end of the fifth day the long axis of the egg (an imaginary line drawn through the center lengthwise) will stand at an angle of 20º from the perpendicular. This angle is increased daily, until at the end of the eighth day it is at about 45º; on the fourteenth day it is 60º; on the twenty-first day it is 75º, while at the end of 4 weeks the egg stands perfectly upright in the liquid, the point or small end downward.
This action is based on the fact that the air cavity in the big end of the egg increases in size and capacity, from day to day, as the egg grows older. An apparatus (originally devised by a German poultry fancier) based on this principle, and by means of which the age of an egg maintained at ordinary temperature may be told approximately to within a day, is made by placing a scale of degrees, drawn from 0º to 90º (the latter representing the perpendicular) behind the vessel con-
[284]
EGGS
taining the solution, and observing the angle made by the axis of the egg with the perpendicular line. This gives the age of the egg with great accuracy.
Weights of Eggs. The following table shows the variation in weight between eggs of the same family of chickens and of the comparative value of the product of different kinds of fowls:
|
|
Weight of Whole Eggs, Grains |
Weight of Shell, Grains |
Net. |
|
Common hen, small |
635.00 |
84.86 |
550.54 |
|
Common hen, mean |
738.35 |
92.58 |
645.77 |
|
Common hen, large |
802.36 |
93.25 |
709.11 |
|
Italian hen |
840.00 |
92.50 |
747.50 |
|
Houdan |
956.60 |
93.50 |
853.10 |
|
La Flesche |
926.50 |
94.25 |
835.25 |
|
Brahma |
1,025.50 |
114.86 |
910.64 |
From this it will be seen that the Houdans and Brahmas are the most profitable producers, as far as food value of the product is concerned provided, of course, they are equally prolific with the ordinary fowl.
Another calculation is the number of eggs to the pound, of the various weights. This is as follows:
Small ordinary eggs (635 grains) 12.20 to pound
Large ordinary eggs (802 grains) 9.25 to pound
Houdan eggs 8.0 to pound
Brahma, mean 7.4 to pound
Brahma, large 7.1 to pound
Dried Yolk of Egg. To prepare this, the yolks of eggs, separated from the whites, are thoroughly mixed with their weight of water. The resulting emulsion is strained and evaporated under reduced pressure at a temperature of 87 to 122º F., to a paste. The latter is further dried over quicklime or a similar absorbent of moisture, at a temperature of 77 to 86º F., and ground to a fine powder.
Egg Oil.
Yolks of eggs (about 250) 5.0 parts
Distilled water 0.3 parts
Beat this together and heat the mass with constant stirring in a dish on the water bath until it thickens and a sample exhibits oil upon pressing between the fingers. Squeeze out between hot plates, mix the turbid oil obtained with 0.05 parts of dehydrated Glauber's salt, shake repeatedly, and finally allow to settle. The oil, which must be decanted clear from the sediment, gives a yield of at least 0.5 parts of egg oil,
Artificial Egg Oil.
Yellow beeswax 0.2 parts
Cacao oil 0.5 parts
Melt on the water bath and gradually add 9 parts of olive oil.
Egg Powder.
Sodium bicarbonate 8 ounces
Tartaric acid 3 ounces
Cream tartar 5 ounces
Turmeric, powdered 3 drachms
Ground rice 16 ounces
Mix and pass through a fine sieve. One teaspoonful to a dessertspoonful
(according to article to be made), to be mixed with each half pound of flour.
The Preservation of Eggs. The spoiling of eggs is due to the entrance of air carrying germs through the shells. Normally the shell has a surface coating of mucilaginous matter, which prevents for a time the entrance of these harmful organisms into the egg. But if this coating is removed or softened by washing or otherwise the keeping quality of the egg is much reduced. These facts explain why many methods of preservation have not been entirely successful, and suggest that the methods employed should be based upon the idea of protecting and rendering more effective the natural coating of the shell, so that air bearing the germs that cause decomposition may be completely excluded.
Eggs are often packed in lime, salt, or other products, or are put in cold storage for winter use, but such eggs are very far from being perfect when they come upon the market. German authorities declare that water glass more closely conforms to the requirements of a good preservative than any of the substances commonly employed. A 10 per cent solution of water glass is said to preserve eggs so effectually that at the end of three and one-half months eggs still appeared to be perfectly fresh. In most packed eggs the yolk settles to one side, and the egg is then inferior in quality. In eggs preserved in water glass the yolk retained its normal position in the egg, and in taste they were not to be distinguished from fresh, unpacked store eggs.
Of twenty methods tested in Germany, the three which proved most effective were coating the eggs with vaseline, preserving them in limewater, and preserving them in water glass. The conclusion was reached that the last is preferable, because varnishing the eggs with vaseline takes considerable time, and treating them with limewater is likely to give the eggs a limy flavor.
[285]
EGGS - EKTOGAN
Other methods follow:
I. Eggs can be preserved for winter use by coating them, when perfectly fresh, with paraffine. As the spores of fungi get into eggs almost as soon as they are laid, it is necessary to rub every egg with chloroform or wrap it a few minutes in a chloroform soaked rag before dipping it into the melted paraffine. If only a trace of the chloroform enters the shell the development of such germs as may have gained access to freshly laid eggs is prevented. The paraffine coating excludes all future contamination from germ-laden air, and with no fungi growing within, they retain their freshness and natural taste.
II. Preserving with Lime. Dissolve in each gallon of water 12 ounces of quicklime, 6 ounces of common salt, 1 drachm of soda, 1/2 drachm saltpeter, 1/2 drachm tartar, and 1 1/2 drachms of borax. The fluid is brought into a barrel and sufficient quicklime to cover the bottom is then poured in. Upon this is placed a layer of eggs, quicklime is again thrown in and so on until the barrel is filled so that the liquor stands about 10 inches deep over the last layer of eggs. The barrel is then covered with a cloth, upon which is scattered some lime.
III. Melt 4 ounces of clear beeswax in a porcelain dish over a gentle fire, and stir in 8 ounces of olive oil. Let the solution of wax in oil cool somewhat, then dip the fresh eggs one by one into it so as to coat every part of the shell. A momentary dip is sufficient, all excess of the mixture being wiped off with a cotton cloth. The oil is absorbed in the shell, the wax hermetically closing all the pores.
IV. The Reinhard method is said to cause such chemical changes in the surface of the eggshell that it is closed up perfectly air-tight and an admittance of air is entirely excluded, even in case of long-continued storing. The eggs are for a short time exposed to the direct action of sulphuric acid, whereby the surface of the eggshell, which consists chiefly of lime carbonate, is transformed into lime sulphate. The dense texture of the surface thus produced forms a complete protection against the access of the outside air, which admits of storing the egg for a very long time, without the contents of the egg suffering any disadvantageous changes regarding taste and odor. The egg does not require any special treatment to prevent cracking on boiling, etc.
Some object to this on the ground that sulphuric acid is a dangerous poison, that might, on occasion, penetrate the shell.
V. Take about half a dozen eggs and place them in a netting (not so many as would chill the water below the boiling point, even for an instant), into a boiling solution of boric acid, withdraw immediately, and pack. Or put up, in oil, carrying 2 per cent or 3 per cent of salicylic acid. Eggs treated in this way are said to taste, after six months, absolutely as fresh as they were when first put up.
The eggs should be as fresh as possible, and should be thoroughly clean before dipping. The philosophy of the process is that the dipping in boiling boric acid solution not only kills all bacteria existing on, or in, the shell and membrane, but reinforces these latter by a very thin layer of coagulated albumen; while the packing in salicylated oil prevents the admission of fresh germs from the atmosphere. Salicylic acid is objected to on the same grounds as sulphuric acid.
VI. Dissolve sodium silicate in boiling water, to about the consistency of a syrup (or about 1 part of the silicate to 3 parts water). The eggs should be as fresh as possible, and must be thoroughly clean. They should be immersed in the solution in such manner that every part of each egg is covered with the liquid, then removed and let dry. If the solution is kept at or near the boiling temperature, the preservative effect is said to be much more certain and to last longer.
EGG CHOCOLATE:
See Beverages.
EGG DYES:
See Dyes.
EGG LEMONADE:
See Beverages, under Lemonade.
EGG PHOSPHATE:
See Beverages.
EGG STAIN REMOVER:
See Gleaning Preparations and Methods.
EGGS, TESTS FOR:
See Foods.
EIKONOGEN DEVELOPER;
See Photography.
EKTOGAN:
See Antiseptics.
[286]
ELECTROPLATING AND ELECTROTYPING
ELAINE SUBSTITUTE.
A substitute for elaine for woolen yarns is obtained by boiling 4 pounds carrageen moss in 25 gallons water for 3 hours. The soda is then put in and the boiling continued for another half hour; 2 pounds fleabane seeds are gradually added, and a little water to make up for the evaporation. After a further 1 1/2 ours boiling, the extract is passed through a fine sieve and well mixed with 25 pounds cottonseed oil, 12 1/2 pounds sweet oil, and 12 1/2 pounds ammonia solution of 0.96 specific gravity. Next day stir in 25 pounds saponified elaine and 13 pounds of odorless petroleum of 0.885 specific gravity. The resulting emulsion keeps well, dissolves perfectly in lukewarm water, and answers its purpose excellently.
ELECTRODEPOSITION PROCESSES:
See Plating.
ELECTROLYSIS IN BOILERS:
See Boiler Compounds.
Electroplating and Electrotyping
(See also Plating.)
PROCESS OF ELECTROPLATING.
First, clean the articles to be plated. To remove grease, warm the pieces before a slow fire of charcoal or coke, or in a dull red stove. Delicate or soldered articles should be boiled in a solution of caustic potash, the latter being dissolved in 10 times its weight of water.
The scouring bath is composed of 100 parts of water to from 5 to 20 parts of sulphuric acid. The articles may be put in hot and should be left in the bath till the surface turns to an ocher red tint.
The articles, after having been cleansed of grease by the potash solution, must be washed in water and rinsed before being scoured. Copper or glass tongs must then be used for moving the articles, as they must not afterwards be handled. For small pieces, suitable earthenware or porcelain strainers may be used.
The next stage is the spent nitric acid bath. This consists of nitric acid weakened by previous use. The articles are left in until the red color disappears, so that after rinsing they show a uniform metallic tint. The rinsing should be thoroughly carried out.
Having been well shaken and drained, the articles are next subjected to the strong nitric acid bath, which is made up as follows:
Nitric acid of 36º Bé. 100 volumes
Chloride of sodium (common salt) 1 volume
Calcined soot (lampblack) 1 volume
The articles must be immersed in this bath for only a few seconds. Avoid overheating or using too cold a bath. They are next rinsed thoroughly with cold water and are again subjected to a strong nitric
acid bath to give them a bright or dull appearance as required.
To produce a bright finish, plunge them for a few seconds (moving them about rapidly at, the same time) in a cold bath of the following composition:
Nitric acid 100 volumes
Sulphuric acid 100 volumes
Chloride of sodium 1 volume
Again rinse thoroughly in cold water. The corresponding bath giving a dull or matt appearance is composed of:
Nitric acid 200 volumes
Sulphuric acid 100 volumes
Sea salt 1 volume
Sulphate of zinc 1 to 5 volumes
The duration of immersion in this bath varies from 5 to 20 minutes, according to the dullness required. Wash with plenty of water. The articles will then have an unpleasant appearance, which will disappear on plunging them for a moment into the brightening bath and rinsing quickly.
The pieces are next treated with the nitrate of mercury bath for a few seconds.
Plain water 10,000 parts
Nitrate of mercury 10 parts
Sulphuric acid 20 parts
It is necessary to stir this bath before using it. For large articles the proportion of mercury should be greater. An article badly cleaned will come out in various shades and lacking its metallic brightness. It is better to throw a spent bath away than attempt to strengthen it.
The various pieces, after having passed through these several processes, are then ready for the plating bath.
A few words on the subject of gilding may not be amiss. Small articles are gilded hot, large ones cold. The cold cyanide of gold and potassium bath is composed as follows:
Distilled water 10,000 parts
Pure cyanide of potassium 200 parts
Pure gold 100 parts
The gold, transformed into chloride, is dissolved in 2,000 parts of water and
[287]
ELECTROPLATING AND ELECTROTYPING
the cyanide in 8,000 parts. The two solutions are then mixed and boiled for half an hour.
The anode must be entirely submerged in the bath, suspended from platinum wires and withdrawn immediately the bath is out of action.
Hot Gold Bath. Zinc, tin, lead, antimony and the alloys of these metals
are better if previously covered with copper.
The following are the formulas for the other metals per 10,000 parts of distilled water:
Crystallized phosphate of soda, 600 parts; alloys rich in copper castings, 500 parts.
Bisulphide of soda, 100 parts; alloys rich in copper, 125 parts.
Pure cyanide of potassium, 10 parts; alloys rich in copper, 5 parts.
Pure gold transformed into chloride, 10 parts; alloys rich in copper, 10 parts.
Dissolve the phosphate of soda hot in 8,000 parts water, let the chloride of gold cool in 1,000 parts water; mix little by little the second solution with the first; dissolve the cyanide and bisulphide in
1,000 parts water and mix this last solution with the other two. The temperature of the bath may vary between 122º and 175º F.
Silvering. For amateurs a bath of 10 parts silver per 1,000 is sufficient. Dissolve 150 parts nitrate of silver, equivalent to 100 parts pure silver, in 10,000 parts of water and add 250 parts pure cyanide of potassium. Stir it up until completely dissolved, and then filter the solution. Silvering is generally effected cold, except in the case of small articles. Iron, steel, zinc, lead, and tin are better if previously copper-plated and then silvered hot. The cleaned articles are first treated in a nitrate of mercury bath, being kept continually in motion.
With excess of current the pieces become gray, and blacken. In the cold bath anodes of platinum or silver should be employed. Old baths are, in this case, preferable to new. They may, if required, be artificially aged by the addition of 1 or 2 parts in 1,000 of liquid ammonia.
If the anode blackens, the bath is too weak. If it becomes white, there is too much current, and the deposit, being too rapid, does not adhere. The deposit may be taken as normal and regular when the anode becomes gray during the passage of the current and white again when it ceases to flow.
The nickel vat should be of glass, porcelain, or earthenware, or a case lined with impermeable gum. The best nickel bath is prepared by dissolving to saturation, in hot distilled water, nickel sulphate and ammonium, free from oxides or alkalies and alkaline earthy metals.
The proportion of salt to dissolve is 1 part, by weight, to 10 of water. Filter after cooling and the bath is then ready for use.
When the bath is ready and the battery set up, the wires from the latter are joined by binding screws to two metal bars resting on the edge of the vat. The bar joined to the positive pole of the battery supports, through the intervention of a nickel-plated copper hook, a plate of nickel, constituting the soluble anode, which restores to the bath the metal deposited on the cathode by the electrolytic action. From the other bar are suspended the articles to be plated.
These latter should be well polished before being put into the bath. To remove all grease, scrub them with brushes soaked in a hot solution of whiting, boiled in water and carbonate of soda.
Copper and its alloys are cleaned well in a few seconds by immersion in a bath composed of 10 parts, by weight, of water, and 1 part of nitric acid. For rough articles, 2 parts water, 1 nitric acid, and 1 sulphuric acid. For steel and polished castings, 100 parts water to 1 sulphuric acid. The articles should remain in the bath until the whole surface is of a uniform gray tint. They are then rubbed with powdered pumice stone till the solid metal appears. Iron and steel castings are left in the bath for three or four hours and then scrubbed with well-sifted sand.
If the current be too strong, the nickel is deposited gray or even black. An hour or so is time enough to render the coat sufficiently thick and in a condition to stand polishing. When the articles are removed from the bath they are washed in water and dried in hot sawdust.
To polish the articles they should be taken in one hand and rubbed rapidly backward and forward on a strip of cloth soaked in polishing powder boiled in water, the cloth being firmly fixed at one end and held in the other hand. The hollow parts are polished by means of cloth pads of various sizes fixed on sticks. These pads must be dipped in the polishing paste when using them. The articles, when well brightened, are washed in water to get rid of the paste and the wool threads, and finally dried in sawdust.
[288]
ELECTROTYPING - EMBALMING FLUIDS
SOME NOTES ON ELECTROTYPING, PLATING, AND GILDING.
The first step in the process is the preparation of the mold. The substance originally used for the construction of this was plaster of Paris. This substance is, however, porous and must be rendered impermeable. The materials most commonly used of later years are stearine, wax, marine glue, gelatin, india rubber, and fusible alloys. With hollow molds it is a good plan to arrange an internal skeleton of platinum, for ultimate connection with the anodes, in order to secure a good electrical contact with all parts of the mold. When covering several pieces at once, it is as well to connect each of them with the negative pole by an iron or lead wire of suitable dimensions.
Having prepared the molds in the usual way by obtaining an impression in the material when soft, and allowing it to set they should be given a metallic coating on their active surfaces of pure powdered plumbago applied with a polishing brush.
For delicate and intricate objects, the wet process is most suitable. It consists in painting the object with two or more coats of nitrate of silver and ultimately reducing it by a solution of phosphorus in bisulphide of carbon.
The plating baths are prepared as follows:
A quantity of water is put in a jar and to it is added from 8 to 10 parts in 100 of sulphuric acid, in small quantities, stirring continually in order to dissipate the heat generated by the admixture of acid and water. Sulphate of copper (bluestone) is then dissolved in the acidulated water at the normal temperature until it will take up no more. The solution is always used cold and must be maintained in a saturated condition by the addition of copper sulphate crystals or suitable anodes.
For use it should be poured into vessels of clay, porcelain, glass, hard brown earthenware, or india rubber. For large baths wood may be used, lined on the interior with an impervious coating of acid-proof cement, india rubber, marine glue, or even varnished lead sheets.
If the solution be too weak and the current on the other hand be too strong, the resulting deposit will be of a black color. If too concentrated a solution and too weak a current be employed, a crystalline deposit is obtained. To insure a perfect result, a happy medium in all things is necessary.
During the process of deposition, the pieces should be moved about in the bath as much as possible in order to preserve the homogeneity of the liquid. If this be not attended to, stratification and circulation of the liquid is produced by the decomposition of the anode, and is rendered visible by the appearance of long, vertical lines on the cathode.
For amateurs and others performing small and occasional experiments, the following simple apparatus will be serviceable. Place the solution of sulphate of copper in an earthenware or porcelain jar, in the center of which is a porous pot containing amalgamated zinc and a solution of sulphuric acid and water, about 2 or 3 parts in 100. At the top of the zinc a brass rod is fixed, supporting a circle of the same metal, the diameter of which is between that of the containing vessel and the porous pot. From this metallic circle the pieces are suspended in such a manner that the parts to be covered are turned toward the porous pot. Two small horsehair bags filled with copper sulphate crystals are suspended in the solution to maintain its
saturation.
ELM TEA.
Powdered slippery elm bark 2 teaspoonfuls
(or the equivalent in whole bar)
Boiling water 1 cup
Sugar, enough.
Lemon juice, enough.
Pour the water upon the bark. When cool, strain and flavor with lemon juice and add sugar. This is soothing in case of inflammation of the mucous membrane.
EMBALMING FLUIDS.
Success in the use of any embalming fluid depends largely on manipulation, an important part of the process being the thorough removal of fluid from the circulatory system before undertaking the injection of the embalming liquid.
I.
Solution zinc chloride (U.S.P.) 1 gallon
Solution sodium chloride 6 ounces
to pint. 6 pints
Solution mercury bichloride,
1 ounce to pint 4 pints
Alcohol 4 pints
Carbolic acid (pure) 8 ounces
Glycerine 24 fluidounces
[289]
EMBALMING FLUIDS - EMULSIFIERS
Mix the glycerine and carbolic acid, then all the other ingredients, when a clear solution of 3 gallons results, which is the proper amount for a body weighing 150 pounds.
II.
Arsenious acid 100 parts
Sodium hydrate 50 parts
Carbolic acid and water, of each a sufficient quantity.
Dissolve the arsenious acid and the soda in 140 parts of water by the aid of heat. When the solution is cold, drop carbolic acid into it until it becomes opalescent, and finally add water until the finished product measures 700 parts.
III.
Salicylic acid 4 drachms
Boric acid 5 drachms
Potassium carbonate 1 drachm
Oil of cinnamon 3 drachms
Oil of cloves 3 drachms
Glycerine 5 ounces
Alcohol 12 ounces
Hot water 12 ounces
Dissolve the first 3 ingredients in the water and glycerine, the oils in the alcohol, and mix the solutions.
IV.
Thymol 15 grains
Alcohol 1/2 ounce
Glycerine 10 ounces
Water 5 ounces
V.
Cooking salt 500 parts
Alum 750 parts
Arsenious acid 350 parts
Zinc chloride 120 parts
Mercury chloride 90 parts
Forma1dehyde solution, 40 per cent 6,000 parts
Water, up to 24,000 parts
VI.
Arsenious acid 360 grains
Mercuric chloride 1 1/4 ounces
Alcohol 9 ounces
Sol. ac. carbolic, 5 per cent 120 ounces
From 10 to 12 pints are injected into the carotid artery at first slowly and afterwards at intervals of from 15 to 30 minutes.
EMERALD (IMITATION):
See Gems, Artificial.
EMERY:
Emery Grinder. Shellac, melted together with emery and fixed to a short
metal rod, forms the grinder used for opening the holes in enameled watch dials and similar work. The grinder is generally rotated with the thumb and forefinger, and water is used to lubricate its cutting part, which soon wears away. The grinder is reshaped by heating the shellac and molding the mass while it is in a plastic condition.
Preparing Emery for Lapping. To prepare emery for lapping screw-gages, plugs, etc., fill a half-pint bottle with machine oil and flour emery, 7 parts oil to 1 part emery, by bulk. Mix thoroughly and let stand for 20 minutes to settle. Take the bottle and pour off one-half the contents without disturbing the settlings. The portion poured off contains only the finest emery and will never scratch the work.
For surface lapping put some flour emery in a linen bag and tie up closely with a string. Dust out the emery by striking the bag against the surface plate; use turpentine for rough lapping and the dry surface plate for finishing.
Removing Glaze from Emery Wheels. If the wheel is not altogether too hard, it can sometimes be remedied by reducing the face of the wheel to about 1/8 inch, or by reducing the speed, or by both. Emery wheels should be turned off so that they will run true before using. A wheel that glazes immediately after it has been turned off, can sometimes be corrected by loosening the nut, and allowing the wheel to assume a slightly different position, when it is again tightened.
Emery Substitute. For making artificial emery, 1,634 parts of the following substances may be employed: Seven hundred and fifty-nine parts of bauxite, 700 parts of coke, and 96 parts of a flux, which may be a carbonate of lime, of potash, or of soda, preferably carbonate of lime on account of its low price. These materials are arranged in alternate layers and fused in an oven having a good draught. They are said to yield an artificial emery similar to the natural emery of Smyrna and Naxos, and at low cost.
EMULSIFIERS:
Rosin Soap as an Emulsifier. The soap should be made by boiling gently for 2 hours, in an evaporating dish, a mixture of 1,800 grains rosin and 300 caustic soda with 20 fluidounces water. Upon cooling, the soap separates as a yellow mass, which is drained from the liquid, squeezed, then heated on a water bath until it is dry and friable. Fixed oils may be emulsified by adding 1 ounce
[290]
EMULSIFIERS - ENAMELING
to a solution of 10 grains soap in 1 ounce water. Volatile oils require 10 grains rosin soap, 2 ounces water, and 2 drachms oil. Creosote requires double this amount of soap. Thymol may be rendered miscible with water by dissolving 18 grains together with 20 grains soap in 3 fluidounces alcohol, then adding enough water to make 6 fluidounces.
Of course many other substances may be emulsified with the same emulsifier.
Yolk of Egg as an Emulsifier. The domestic ointment of Unona, consisting of a mixture of oil and yolk of egg, is miscible in all proportions with water. It is proposed to utilize this fact by substituting a diluted ointment for the gum emulsions in general use, the following being given as a general formula:
Yolk of egg 10 parts
Balsam Peru 1 to 2 parts
Zinc oxide 5 to 10 parts
Distilled water 100 parts
If desired, 33 parts of vinegar may be substituted for the same amount of water, while oil of cade, oil of birch, lianthral or storax may be substituted for the balsam Peru, and an equal quantity of talc, magnesium carbonate, sulphur of bismuth subcarbonate, may be introduced in place of the oxide of zinc. A further variation in the character of the liquid may be introduced by the use of medicated or perfumed waters instead of the plain distilled water. Where so diluted, as in the above formula, the yolk of egg separates out after long standing, but the mixture quickly reemulsifies upon shaking. Tar and balsams can be emulsified by mixing with double their quantity of yolk of egg, then diluting by the addition of small quantities of water or milk.
Emulgen. This emulsifying agent has the foil owing composition: Gluten, 5; gum acacia, 5; gum tragacanth, 20; glycerine, 20; water, 50; alcohol, 10. This mixture forms a clear grayish jelly.
EMULSIONS OF PETROLEUM:
See Petroleum.
Enameling
(See also Ceramics, Glazes, Paints,
Waterproofing, and Varnishes.)
COMMERCIAL ENAMELING.
Commercial enameling includes: (1) Hollow ware enameling for domestic use; (2) hollow ware enameling for chemical use; (3) enameling locomotive and other tubes; (4) enameling drain and water pipes; (5) signboard enameling.
There is one defect to which all enamel ware is subject, and that is chipping. This may be caused by (1) imperfect mixing of the enamels; (2) imperfect fusing; (3) imperfect pickling of the iron; (4) rough usage. With ordinary care a well-enameled article has been known to last in daily use for 10 or 12 years, whereas defective enameling, say, on a sign tablet which is exempt from rough usage may not have a life exceeding a few months. All enameled articles, such as hollow ware and sign tablets, first receive a coating of a composition chiefly composed of glass called "gray," and this is followed by a deposit of "white," any additional color required being laid above the white. In the mixing and depositing of these mixtures lie the secrets of successful enameling. The "gray" has to be fused not only on but also into the metal at a bright red almost white heat, and it is obvious that its constituents must be arranged and proportioned to expand and contract in a somewhat uniform manner with the iron itself. The "white" has to be fused on the surface of the gray, but the gray being much harder is not affected by the second firing. If it were liquid it would become mixed with the white and destroy its purity. Frequently, owing to inferior chemicals, imperfect mixing or fusing, a second coating of white is necessary, in order to produce a surface of the necessary purity and luster. The difficulties of enameling are thus easily understood. Unless the metals and chemicals are so arranged and manipulated that their capacities of expansion and contraction are approximately the same, inferior work will be produced. Oxide of iron on the surface of the plates, inferior chemicals, incorrect mixings, insufficient or overheating in the process of fusing, prevent that chemical combination which is essential to successful enameling. The coatings will be laid on and not combined, with the result that there will be inequalities in expansion and contraction which will cause the
enamel to chip off immediately if submitted to anything approaching rough usage, and in a very short time if submitted to chemical or ordinary atmospheric conditions.
The manufacture of sign tablets is the simplest form to which this important art is adapted. Sign-tablet enameling is, however, kept as great a secret as any other type. This branch of the industry
[291]
ENAMELING
is divided up as follows: (1) Setting the plates; (2) scaling and pickling the plates; (3) mixing the enamel constituents; (4) melting the enamel constituents; (5) grinding the enamel constituents; (6) applying the enamel; (7) drying the enamel coatings; (8) fusing the enamel on the articles; (9) lettering including alphabetical and other drawing, spacing, and artistic art in arrangement; (10) stencil cutting on paper and stencil metal; (11) brushing; (12) refusing. Distinctive branches of this work have distinctive experts, the arrangement being generally as follows: Nos. 1 and 2 may or may not be combined; Nos. 3 and 5 may or may not be combined; Nos. 4, 7, 8, and 12 generally combined; No. 6 generally the work of girls; Nos. 9 and 10 generally combined; No. 11 generally the work of girls and boys. The twelve processes, therefore, require six classes of trained work people, and incompetence or carelessness at any section can only result in imperfect plates or "wasters."
A brief description of these processes will enable the reader to understand the more detailed and technical description to follow, and is, therefore, not out of place. Ordinary iron sheets will do for the manufacture of sign tablets; but a specially prepared charcoal plate can be had at a slightly increased price. The latter type is the best, for in many cases the scaling and pickling may, to a certain extent, be dispensed with. To make this article, however, as complete as possible, we shall begin from the lowest rung of the manufacturing ladder i.e., from the first steps in the working of suitable iron.
I. Setting. The plates may be received in sheets, and cut to the required size at the enameling factory, or, what is more general, received in sizes according to specification. The former are more liable to have buckled slightly or become dented, and have to be restored to a smooth and uniform surface by hammering on a flat plate. The operation seems simple, but an inexperienced operator may entirely fail to produce the desired result, and, if he does succeed, it is with the expenditure of a great amount of time. An expert setter with comparatively few and well-directed strokes brings an imperfect plate into truth and in readiness for the next operation.
II. Scaling and Pickling. The annealing of the sheets in special furnaces loosens the scale, which can then be easily removed, after which immersion for some time in diluted sulphuric or muriatic acid thoroughly cleans the plate. Firing to a red heat follows, and then a generous course of scrubbing, and the last traces of acid are removed by dipping in boiling soda solution. Scouring with sand and washing in clean water may follow, and the metal has then a perfect and chemically clean surface.
III. Mixing the Enamel Constituents. Ground, foundation, or gray.
All articles, whether hollow ware or plates, are operated upon in a very similar manner. Both require the foundation coating generally called "gray." The gray constituents vary considerably in different manufactures; but as regards the use of lead, it is universally conceded that while it may in many instances be used with advantage in the enameling of sign tablets, etc., it should under no circumstances be introduced into the coating of articles for culinary purposes, or in which acids are to be used. The first successful commercial composition of this covering was: Gullet (broken glass), carbonate of soda, and
boracic acid. This composition remained constant for many years, but ultimately gave place to the following: Gullet, red lead, borax, niter. The borax and red lead form the fluxes, while the niter is to "purify" the mass. Some of the later mixings consist of the following: Silica powder, crystallized or calcium borax, white lead, fused together.
This would be called a frit, and with it should be pulverized powdered silica, clay, magnesia. This recipe is one requiring a very high temperature for fusing: Silica powder, borax, fused and ground with silica, clay, magnesia. This requires a slightly lower temperature:
Frit of silica powder, borax, feldspar, fused together, and then ground with,clay, feldspar, and magnesia.
The approximate quantities of each constituent will be given later, but it must always be remembered that no hard-and-fast line can be laid down. Chemicals vary in purity, the furnaces vary in temperature, the pounding, grinding, and mixing are not always done alike, and each of these exerts a certain influence on the character of the "melt."
These compositions may be applied to the metal either in the form of a powder or of a liquid. Some few years ago the powder coating was in general use, but at the present time the liquid form is in favor, as it is considered easier of application, capable of giving a coating more uniform in thickness and less costly. In using the powder coating the plate is rubbed with a cloth dipped in a gum
[292]
ENAMELING
solution, and the powder then carefully dusted through a sieve over the surface. In this condition the plate is submitted to the fusing process. In using the liquid material the plate surface is dipped into or has the liquid mixing carefully poured over it, any surplus being drained off, and any parts which are not to be coated being wiped clean by a cloth. The coating is then dried in suitable stoves, after which it is ready for fusing on to the iron. The gray coating should be fairly uniform and smooth, free from holes or blisters, and thoroughly covering every part of the iron which is to be subjected to any outside influence. Cooling slowly is important. Rapid cooling frequently causes chipping of the coating, and in any case it will greatly reduce the tenacity of the connection existing between the glaze and the metal.
Generally the next surface is a white one, and it depends upon the class of article, the character of the enamels, and the efficiency of application, whether one coat or two will be required. Roughly speaking, the coating is composed of a glass to which is added oxide of tin, oxide of lead, or some other suitable opaque white chemical. The mixture must be so constituted as to fuse at a lower temperature than the foundation covering. If its temperature of fusion were the same the result would be that the gray would melt on the iron and become incorporated with the white, thus loosening the attachment of the mass to the iron and also destroying the purity of the white itself. Bone ash is sometimes used, as it becomes uniformly distributed throughout the melt, and remains in suspension instead of settling. Bone ash and oxide of lead are, however, in much less demand than oxide of tin.
The lead is especially falling into disfavor, for the following reasons: Firstly, it requires special and laborious treatment; secondly, it gives a yellowish-white color; thirdly, it cannot resist the action of acids. The following is a recipe which was in very general use for some years: Glass (cullet), powdered flint, lead, soda (crystals), niter, arsenic. Another consists of the folio wing: Borax,
glass, silica powder, oxide of tin, niter, soda, magnesia, clay. These are fused together, and when being ground a mixture of Nos. 1, 3, 7, and boracic acid is added.
Enamel mixings containing glass or china are now generally in use, although for several years the experience of manufacturers using glass was not satisfactory Improved compositions and working now make this constituent a most useful, and, in fact, an almost essential element. The glass should be white broken glass, and as uniform in character as possible, as colored glass would impart a tinge of its own color to the
mixing.
The following are two distinct glazes which do not contain glass or porcelain: Feldspar, oxide of tin, niter, soda. This is free from any poisonous body and requires no additions: Silica powder, oxide of tin, borax, soda, niter, carbonate of ammonia, or magnesia.
Alkalies. Of the alkalies which are necessary to produce complete fusion of and combination with the quartz, soda is chiefly applied in enamel manufactures, as the fusing temperature is then lower.
Bone Ash. This material will not add opacity, but only semi-transparency to the enamel, and is therefore not much used.
Boracic Acid. Boracic acid is sometimes substituted for silicic acid, but generally about 15 per cent of the former to 85 per cent of the latter is added. Borax as a flux is, however, much more easily used and is therefore largely employed in enamel factories.
Borax. Calcined borax, that is, borax from which a large proportion of the natural moisture has been eliminated, is best for enamel purposes. It is a flux that melts at medium heat, and enters into the formation of the vitreous basis. Borax has also the property of thoroughly distributing oxide colors in the enamels.
Clay. Only a fairly pure clay can be used in enamel mixings, and the varieties of clay available are therefore limited. The two best are pipe or white clay and china clay kaolin. The latter is purer than the former, and in addition to acting as a flux, it is used to increase the viscosity of mixings and therefore the opacity. It is used in much the same way as oxide of tin.
Cryolite. Ground cryolite is a white mineral, easily fusible, and sometimes used in enamel mixings. It is closely associated with aluminum.
Cullet. This is the general material used as a basis. Clear glass only should be introduced; and as the compositions of glass vary greatly, small experimental frits should always be made to arrive at the correct quantity to be added.
Feldspar. The introduction of feldspar into an enamel frit increases consistency. The common white variety is
[293]
ENAMELING
generally used, and its preliminary treatment by pounding is similar to that adopted with quartz.
Fluor-Spar. In this mineral we have another flux, which fuses at a red heat.
Fluxes. These are for the purpose of regulating the temperature of fusion of a mixing frit some being better adapted for this purpose than others. This, however, is not the only consideration, for the character of the flux depends upon the composition or chemical changes to which the ingredients are to be subjected. The fluxes are borax, clays, cullet, porcelain, feldspar, gypsum, and fluorspar.
Glass. Glass is composed of lime, silicic acid, and soda or potash. The use of the glass is to form the hard, crystal-like foundation.
Gypsum. This mineral is sometimes used in conjunction with baryta and
fluor-spar.
Lead. Crystallized carbonate of lead, or "lead white," is frequently used in enamels when a low temperature for fusion is required. It should never be used on articles to be submitted to chemical action, or for culinary use. Minium is a specially prepared oxide of lead, and suitable for enameling purposes, but is expensive.
Lime. Lime is in the form of carbonate of calcium when used.
Magnesium Carbonate is used only in small quantities in enamel mixings. It necessitates a higher temperature for fusion, but does not affect the color to the slightest extent if pure.
Manganese. As a decolorant, this mineral is very powerful, and therefore only small quantities must be used. Purity of the mineral is essential i.e, it should contain from 95 to 98 per cent of binoxide of manganese.
Niter. At a certain temperature niter shows a chemical change, which, when affected by some of the other constituents, assists in the formation of the vitreous base.
Porcelain. Broken uncolored porcelain is sometimes used in enamel manufacture. Its composition: Quartz, china clay, and feldspar. It increases viscosity.
Red Lead. This decolorant is sometimes called purifier. It will, however, interfere with certain coloring media, and when this is the case its use should at once be discontinued.
Silicic Acid. Quartz, sand, rock crystal, and flint stone are all forms of this acid in crystallized form. By itself it is practically infusible, but it can be incorporated with other materials to form mixings requiring varying temperatures for fusion.
Soda. The soda in general use is carbonate of soda 58 per cent or enameling soda. The latter is specially prepared, so as to free it almost entirely from iron, and admit of the production of a pure white enamel when such is required.
Tin Oxide. All enamels must contain white ingredients to produce opacity, and the most generally used is oxide of tin. By itself it cannot be fused, but with proper manipulation it becomes diffused throughout the enamel mass. On the quantity added depends the denseness or degree of opacity imparted to the enamel.
It will be understood that the enamel constituents are divided into four distinct groups: I. Fundamental media. II. Flux media. III. Decolorant media. IV. Coloring media. We have briefly considered the three first named, and we will now proceed to No. IV. The coloring material used is in every case a metallic oxide, so that, so far as this goes, the coloring of an enamel frit is easy enough. Great care is, however, necessary, and at times many difficulties present themselves, which can only be overcome by experience. Coloring oxides are very frequently adulterated, and certain kinds of the adulterants are injurious to the frit and to the finish of the color.
Comparison of Hollow Ware and Sign Tablet Enameling. The enameling for sign tablets is much the same as for hollow ware; the mixings are practically alike, but, as a general rule, the mixing is applied in a much more liquid form on the latter. It is easy to understand that hollow ware in everyday use receives rougher usage than tablets. By handling, it is submitted to compression, expansion, and more or less violence due to falls, knocks, etc., and unless, therefore, the enamel coating follows the changes of the metal due to these causes, the connection between the two will become loosened and chipping will take place.
The enamel, therefore, though much alike for both purposes, should be so prepared for hollow ware that it will be capable of withstanding the changes to which we have referred. In all cases it must be remembered that the thinner the coat of the enamel the better it will be
[294]
ENAMELING
distributed over the iron, and the greater will be its adherence to the iron. Any article heavily enameled is always liable to chip, especially if submitted to the slightest bending action, and therefore any excess of material added to a plate means that it will always be readily liable to separate from the plate. In hollow-ware enameling the preparation of each frit generally receives somewhat more attention than for plate enameling. The grinding is more effectively carried out, in order to remove almost every possibility of roughness on any part of the surface, especially the inside surface.
The iron used in tablet and hollow ware manufacture is rolled sheet iron. It is supplied in a variety of qualities. Charcoal iron is purer than ordinary plate iron, more ductile, and therefore capable of being driven out to various forms and depths by stamping presses. The surface of the charcoal iron is not so liable to become oxidized, and therefore can be more readily made chemically clean for the reception of the enamels. Some manufacturers use charcoal plates for tablet work, but these are expensive; the ordinary plates, carefully pickled and cleaned, adapt themselves to the work satisfactorily.
The sheet irons generally used for the enameling purposes referred to vary in gauge. The finer the iron the greater must be the care used in coating it with enamel. Thin iron will rapidly become hot or cool, the temperatures changing much more quickly than that of the mixing. Unless care, therefore, is used, the result of fusing will be that the enamel mass will not have become thoroughly liquid, and its adherence to the iron will be imperfect.
If, however, the temperature is gradually raised to the maximum, and sympathetic combination takes place, the dangers of rapid cooling are avoided. Again, the iron, in losing its temperature more rapidly than the enamel, will contract, thus loosening its contact with the glaze, and the latter will either then, or after a short period of usage, chip off. We then arrive at the following hard-and-fast rules: (1) In all classes of enameling, but particularly where thin iron sheets are used, the temperature of the plate and its covering must be raised very gradually and very uniformly. (2) In all cases a plate which has had a glaze fused on its surface must be cooled very gradually and very uniformly. The importance of these rules cannot be over-estimated, and will, therefore, be referred to in a more practical way later.
In enameling factories no causes are more prolific in the production of waste than these, and in many cases the defects produced are erroneously attributed to something else. Cast iron is much easier to enamel than wrought iron. This is due to the granular character of its composition. It retains the enamels in its small microscopic recesses, and greater uniformity can be arrived at with greater ease. Cast-iron enameled sign tablets and hollow ware were at one time made, but their great weight made it impossible for them ever to come into general use.
Wrought-iron plates, if examined microscopically, will show that they are of a fibrous structure, the fibers running in the direction in which they have been rolled. The enamels, therefore, will be more liable to flow longitudinally than transversely, and this tendency will be more accentuated at some places than at others. This, however, is prevented by giving the iron sheets what might be described as a cast-iron finish. The sheets to be enameled should be thoroughly scoured in all directions by quartz or flint sand, no part of the surface being neglected. This thorough scrubbing will roughen the surface sufficiently to make it uniformly retentive of enamel mixture, and in no cases should it be omitted or carelessly carried out.
Copper Enameling. On a clean copper surface the enameling process is easy. The foundation glaze is not essential, and when required the most beautiful results of blended colors can be obtained by very little additional experience to ordinary enameling.
When the vase or other article has been hammered out to the required shape in copper, it is passed on to another class of artisans, who prepare it for the hands of the enameler. The design or designs are sketched carefully. The working appliances consist only of a pointed tool, two or three small punches of varying sizes, and a hammer. With this small equipment the operator sets to work. The spaces between each dividing line are gradually lowered by hammering, and when this has been uniformly completed, each little recess is ready to receive its allotment of enamel. More accurate work even than this can be obtained by the introduction of flat wire. This wire is soldered or fixed on the vase, and forms the outline for the entire design. It may be of brass, copper, or gold, but is fixed and built round every item of the whole design with the most
[295]
ENAMELING
laborious care. It stands above the surface of the design on the copper articles, but the little recesses formed by it are then gradually filled up by enamel in successive fusings. The whole surface of the article is now ground perfectly smooth and polished until its luster is raised to the highest point possible, and when this stage has been reached the article is ready for the market.
From the Sheet to the Sign Tablet. The plates are generally in lengths of 6 feet by 2 feet, 6 feet by 3 feet, etc., the gauge generally being from 14 to 22, according to the size and class of plates to be enameled. These must be cut, but some enamelers prefer to order their plates in specified sizes, which does away with the necessity of cutting at the enameling factory. In order, however, to make this article complete, we will assume that a stock of large plates is kept on hand, the sizes being 6 feet by 3 feet and 6 feet by 2 feet. An order for sign tablets is given; particulars, say as follows: Length, 2 feet by 12 inches, white letters on blue ground; lettering, The Engineer, 33 Norfolk Street; block letters, no border line, 2 holes. For ordinary purposes these particulars would be sufficient for the enameler.
Stage I. Cutting the plate is the first operation. The plates 6 feet by 2 feet would first be cut down the center in a circular cutting machine, thus forming two strips, 6 feet by 12 inches. Each strip would then be cut into three lengths of 2 feet each. If a guillotine had to be used instead of a circular cutter, the plate would be first cut transversely at distances of 2 feet, thus forming three square pieces of 2 feet by 2 feet. These would then be subdivided longitudinally into two lengths each, the pieces being then 2 feet by 12 inches. Each sheet would thus be cut into six plates.
Stage II. The cut plates should next have any roughness removed from the edges, then punched with two holes one at each end, followed by leveling or setting. This is done by hammering carefully on a true flat surface.
Stage III. The plates should then be taken and dipped into a hydrochloric acid bath made up of equal quantities of the acid and water. The plates are then raised to a red heat in the stoves, and on removal it will be found that the scale iron oxide has become loosened, and will readily fall off, leaving a clean metallic surface. A second course of cleaning then follows in diluted sulphuric acid 1 part acid to 20 parts water. In this bath the iron may be kept for about 12 hours. In some cases a much stronger bath is used, and the plates are left in only a very short time. The bath is constructed of hard wood coated inside with suitable varnish.
In mixing the sulphuric acid bath it must be remembered that the acid should be slowly poured into the water under continuous stirring. Following the bath, the metal is rinsed in water, after which it is thoroughly scoured with fine flinty sand. Rinsing again follows, but in boiling water, and then the metal is allowed to dry. The enameling process should immediately follow the drying, for if kept for any length of time the surface of the metal again becomes oxidized. In hollow-ware enameling the hydrochloric acid bath may be omitted.
Stage IV. The plates are now ready for the reception of the foundation or gray coating. If powder is used the plate is wiped over with a gum solution, and then the powder is carefully and uniformly dusted through a fine sieve over the surface. The plate is then reversed and the operation repeated on the other side. If a liquid "gray" is to be used it should have a consistency of cream, and be poured or brushed with equal care over the two surfaces in succession, after the plate has been heated to be only just bearable to the touch. The plates are then put on rests, or petits, in a drying stove heated to about 160º F., and when thoroughly dry they are ready for the fusing operation. The petits, with the plates, are placed on a long fork fixed on a wagon, which can be moved backward and forward on rails; the door of the fusing oven is then raised and the wagon moved forward. The fork enters the oven just above fire clay brick supports arranged to receive the petits. The fork is then withdrawn and the door closed. The stove has a cherry-red, almost white, heat and in a few minutes the enamel coating has been uniformly melted, and the plates are ready to be removed on the petits and fork in the same manner as they were inserted. Rapid cooling must now be carefully avoided, otherwise the enamel and the iron will be liable to separate, and chipping will result. The temperature of fusion should be about 2,192º F.* When all the plates have been thus prepared they are carefully examined and defective ones laid aside, the others being now ready for the next operation.
* Melting a piece of copper will approximately represent this temperature.
[296]
ENAMELING
Stage V. The coating of the plate with white is the next stage. The temperature of fusion of the white glaze is lower than that of the gray, so that the plate will remain a shorter time in the stove, or be submitted to a somewhat lower temperature. The latter system is to be strongly recommended in order to prevent any possibility of fusion of the ground mass. The white should be made as liquid as possible consistent with good results. The advantages of thin coatings have already been explained, but if the mixing is too thin the ground coating will not only be irregularly covered, but, in fusion, bubbles will be produced, owing to the steam escaping, and these are fatal to the sale of any kind of enameled ware. When the plate has been thoroughly dried and fusion has taken place, slow and steady cooling is absolutely essential. Special muffles are frequently built for this purpose, and their use is the means of preventing a large number of wasters. Before putting on the glaze, care must be taken to remove the gray from any part which is not to be coated. The temperature of fusion should be about 1,890º F.,* and the time taken is about 5 minutes.
Stage VI. The stencil must be cut with perfect exactitude. The letters should be as clear as possible, proportioned, and spaced to obtain the best effects as regards boldness and appearance. Stencils may be cut either from paper or from specially prepared soft metal, called stencil metal. The former are satisfactory enough when only a few plates are required from one stencil, but when large quantities are required, say,
60 upward, metal stencils should be used. The paper should be thick, tough, and strong, and is prepared in the following manner: Shellac is dissolved in methylated spirits to the ordinary liquid gum form, and this is spread over both sides of the paper with a brush. When thoroughly dry a second protective coating is added, and the paper is then ready for stencil work. The stencil cutter's outfit consists of suitable knives, steel rule, scales of various fractions to an inch, a large sheet of glass on which the cutting is done, and alphabets and numerals of various characters and types. For ordinary lettering one stencil is enough, but for more intricate designs 2, 3, and even 4 stencils may be required. In the preparation of the plates referred to in the paragraph preceding Stage I, only 1
stencil would be necessary. The paper before preparation would be measured out to the exact size of the plate, and the letters would be drawn in. The cutting would then be done, and the result shown at Fig. 1 would be obtained, the

black parts being cut out. The lines or corners of each letter or figure should be perfectly clear and clean, for any flaw in
the stencil will be reproduced on the plate.
Stage VII. The next stage is the application of the blue enamel. The operation is almost identical with that of the white, but when the coating has been applied and dried, the lettering must be brushed out before it is fused. The coating is generally applied by a badger brush after a little gum water has been added; the effect of this is to make the blue more compact.
Stage VIII. The next operation is brushing; the stencil is carefully placed over the plate, and held in position, and with a small hand brush with hard bristles the stencil is brushed over. This brushing removes all the blue coating, which shows the lettering and leaves the rest of the white intact. When this has been done, the stencil is removed and the connecting ribs of the lettering some of which are marked X in Fig. 2 are then removed by hand, the instrument generally being a pointed stick of box or other similar wood.
Stage IX. Fusing follows as in the case of the white glaze, and the plate is complete. One coat of blue should be sufficient, but if any defects are apparent a second layer is necessary.
The white and blue glazes are applied only on the front side of the plate, the back side being left coated with gray only.
From the Sheet to the Hollow Ware. In hollow-ware enameling, the iron is received in squares, circles, or oblongs, of the size required for the ware to be turned out. It is soft and ductile, and by means of suitable punches and dies it is driven in a stamping press to the necessary shape. For shallow articles only one operation is necessary, but for deeper articles from 2 to 6 operations may be
* Melting a piece of brass will represent this temperature.
[297]
ENAMELING
required, annealing in a specially constructed furnace taking place between each. Following the "drawing" operations comes that of trimming; this may be done in a press or spinning lathe, the object being to trim the edges and remove all roughness. The articles are now ready for enameling. For explanation, let us suppose they are tumblers, to be white inside, and blue outside. The gray is first laid on, then the white, and lastly the blue that is, after the pickling and cleaning operations have been performed. The line of demarcation between the blue, and white must be clear, otherwise the appearance of the article will not be satisfactory. The process of enameling is exactly the same as for sign-plate enameling, but more care must be exercised in order to obtain a smoother surface. While the liquid enamels are being applied, circular articles should be steadily rotated in order to let the coating flow uniformly and prevent thick and thin places. The enameling of "whole drawn" ironware presents no difficulty to the ordinary enameler, but with articles which are seamed or riveted, special care and experience is necessary.
Seamed or riveted parts are, of course, thicker than the ordinary plate, will expand and contract differently, will take longer to heat and longer to cool, and the conclusion, therefore, that must be arrived at is that the thickness should be reduced as much as possible, and the joints be made as smooth as possible. Unless special precautions are taken, cracks will be seen on articles of this kind running in straight lines from the rivets or seams. To avoid these, the enamel liquid must be reduced to the greatest stage of liquidity, the heat must be raised slowly, and in cooling the articles should pass through, say, 2 or 3 muffles, each one having a lower temperature than the preceding one. It is now generally conceded that the slower and more uniform the cooling process, the greater will be the durability of the enamel. Feldspar is an almost absolutely necessary addition to the gray in successful hollow-ware enameling, and the compositions of both gray and white should be such as to demand a high temperature for fusion. The utensils with the gray coating should first be raised to almost a red heat in a muffle, and then placed in a furnace raised to a white heat. The white should be treated similarly, and in this way the time taken for complete fusion at the last stage will be about 4 minutes.
The outside enamel on utensils is less viscous than the inside enamel, and should also be applied as thinly as possible.
Stoves and Furnaces. Fritting and Fusing. The best results are obtained
in enameling when the thoroughly ground and mixed constituents are fused together, reground, and then applied to the metal surface. In cheap enamels the gray is sometimes applied without being previously melted, but it lacks the durability which is obtained by thorough fusion and regrinding. In smelting enamel one of two kinds of furnaces may be used, viz., tank or crucible. The former is better adapted to the melting of considerable quantities of ordinary enamel, while the latter is more suitable for smaller quantities or for finer enamels as the mixture is protected from the direct action of the flames by covers on the crucibles. The number of tanks and crucibles in connection with each furnace depends upon the heating capacity of the furnace and upon the out-turn required. They are so arranged that all or any of them can be used or put out of use readily by means of valves and dampers. Generally, they are arranged in groups of from 6 to 12, placed in a straight or circular line, but the object aimed at is complete combustion of the fuel, and the utilization of the heat to the fullest extent. One arrangement is to have the flame pass along the bottom and sides of the tank and then over the top to the chimney.
The general system in use is, however, the crucible system. The crucibles are made from the best fire clay, and the most satisfactory are sold under the name of "Hessian crucibles." The chief objection to the use of the crucibles is that of cost. They are expensive, and in many factories the life of the crucible is very short, in some cases not extending beyond one period of fusion. When this, however, is the rule rather than the exception, the results are due to carelessness. Sudden heating or cooling of the crucible will cause it to crack or fall to pieces, but for this there is no excuse. Running the molten material quickly out of the crucible and replacing it hurriedly with a fresh cold mixing is liable - in fact, almost certain - to produce fracture, not only causing the destruction of the crucible, but also the loss of the mixing. New crucibles should be thoroughly dried in a gentle heat for some days and then gradually raised to the requisite temperature which they
[298]
ENAMELING
must sustain for the purposes of fusion. Sometimes unglazed porcelain crucibles specially prepared with a large proportion of china clay are used. These are, however, expensive and require special attention during the first melt. The life of all crucibles can be lengthened by:
(1) Gradually heating them before putting them into the fire; (2) never replacing a frit with a cold mass for the succeeding one; it should first be heated in a stove and then introduced into the crucible; (3) carefully protecting the hot crucibles from cold draughts or rapid cooling.
Melting and Melting Furnaces. The arrangement of the melting furnace must be such as to protect the whole of the crucible from chills. The usual pit furnaces, with slight modifications, are suitable for this purpose. The crucible shown at b in Fig. 3 is of the type already
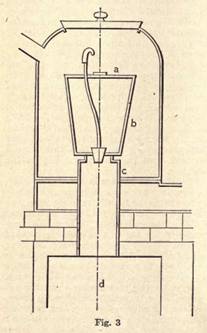
described; at the top it is fitted with a lid, a, hinged at the middle, and at the bottom it is pierced by a 2-inch conical hole.* The hole, while melting is going on, is plugged up with a specially prepared stopper. The crucible stands on a tubular fireproof support, c, which allows the molten mass to be easily run off into a tub of water, which is placed in the chamber, d. The fuel is thrown in from the top, and the supply must be kept uniform. From 4 to 6 of these furnaces are connected with the same chimney; but before passing to the chimney the hot gases are in some cases used for heating purposes in connection with the drying stove. The plug used may be either a permanent iron one coated with a very hard enamel or made from a composition of quartz powder and water. An uncovered iron plug would be unsuitable owing to the action of the iron on the ingredients of the mixing.
In some cases only a very small hole is made in the crucible and no stopper used, the fusion of the mixing automatically closing up the hole. In some other factories no hole is made in the crucible, and when fusion is complete the crucible is removed and the mixing poured out. The two latter systems are bad; in the first there is always some waste of material through leakage, and in the latter the operation of removing the crucible is clumsy and difficult, while the exposure to the colder atmosphere frequently causes rupture.
The plug used should be connected with a rod, as shown in Fig. 3, which passes through a slot in one-half of the hinged lid, a. When fusion is complete this half is turned over, and the plug pulled up, thus allowing the molten mass to fall through into the vat of water placed underneath. The mixing in the crucibles, as it becomes molten, settles down, and more material can then be added until the crucible is nearly full. If the mixing is correctly composed, and has been thoroughly fused, it should flow freely from the crucible when the plug is withdrawn. Fusing generally requires only to be done once, but for fine enamels the operation may be repeated. The running off into the water is necessary in order to make the mass brittle and easy to grind. If this was not done it would again form into hard flinty lumps and require much time and labor to reduce to a powder.
A careful record should be kept of the loss in weight of the dried material at each operation. The weighings should be made at the following points: (1) Before and after melting; (2) after crushing.
The time required for melting varies greatly, but from 6 to 9 hours may be considered as the extreme limits. Gas is much used for raising the necessary heat for melting. The generator may be
* Two inches for gray, one inch for glaze; the hole should be wider at the top
[299]
ENAMELING
placed in any convenient position, but a very good system is to have it in the center of a battery of muffles, any or all of which can be brought into use. When quartz stoppers are used there is considerable trouble in their preparation, and as each new batch of material requires a fresh stopper, wrought-iron stoppers have been introduced in many factories. These are coated with an enamel requiring a much higher temperature of fusion than the fundamental substance, and this coating prevents the iron having any injurious action on the frit.
Fusing. For fusing the enamel muffle furnaces are used; these furnaces are simple in construction, being designed specially for: (1) Minimum consumption of fuel; (2) maximum heat in the muffle; (3) protection of the inside of the muffle from dust, draughts, etc.
The muffle furnaces may be of any size, but in order to economize fuel, it is obvious that they should be no larger than is necessary for the class and quantity of work being turned out. For sign-plate enameling the interior of the muffle may be as much as 10 feet by 5 feet wide by 3 feet in height, but a furnace of this kind would be absolutely ruinous for a concern where only about a dozen small hollow-ware articles were enameled at a time. The best system is to have 2 or 3 muffle furnaces of different dimensions, as in this way all or any one of them can be brought into use as the character and number of the articles may require. The temperature throughout the muffle is not uniform, the end next to the furnace being hotter than that next to the door. In plate enameling it is therefore necessary that the plates should be turned so that uniform fusion of the enamel may take place. In the working of hollow ware the articles should be first placed at the front of the muffle and then moved toward the back. The front of the furnace is closed in by a vertically sliding door or lid, and in this an aperture is cut, through which the process of fusion can be inspected. All openings to the muffle should be used as little as possible; otherwise cold air is admitted, and the inside temperature rapidly lowered.
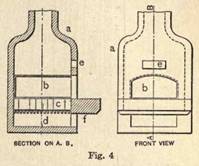
Fig. 4 shows a simple arrangement of a muffle furnace; a is the furnace itself, with an opening, e, through which the fuel is fed; b is the muffle; c shows the firebars, and d the cinder box; f is a rest or plate on which is placed the articles to be enameled. The plate or petits on which the articles rest while being put into the muffle should be almost red hot, as the whole heat of the muffle in this way begins to act immediately on the enamel coating. The articles inside the muffles can be moved about when necessary, either by a hook or a pair of tongs, but care must be taken that every part of the vessel or plate is submitted to the same amount of heat.
In Figs. 5, 6, and 7 are given drawings of an arrangement of furnaces, etc., connected with an enameling factory at
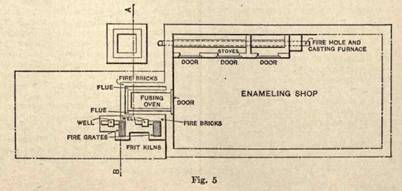
[300]
ENAMELING
present working. The stoves shown in Fig. 5 are drying stoves fired from the end by charcoal, and having a temperature of about 160º F. Fig. 6 shows the arrangement of the flues for the passage of the gases round the fusing oven. The section through the line A B, Fig. 5, as shown in Fig. 7, and the section through
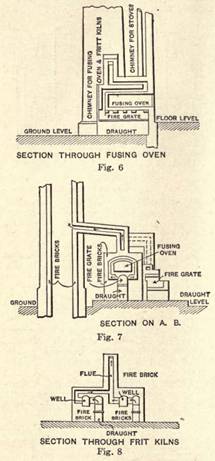
the frit kilns, as shown in Fig. 8, are sufficiently explanatory. The frit kilns and the fusing oven flues both lead to the brick chimney, but the stoves are connected to a wrought-iron chimney shown in Fig. 6. Another arrangement would have been to so arrange the stoves that the gases from the frit kilns could have been utilized for heating purposes.
Fuel. The consumption of fuel in an enameling factory is the most serious item of the expenditure. Ill-constructed or badly proportioned stoves may represent any loss of coal from a quarter to one ton per day, and as great and uniform temperatures must be maintained, fuel of low quality and price is not desirable. In the melting stoves either arranged as tank or crucible furnaces, the character of the coal must not be neglected, as light dust, iron oxide, or injurious gases will enter into the crucibles through any opening, especially if the draught is not very great. Almost any of the various kinds of fuel may be used, provided that the system of combustion is specially arranged for in the construction of the furnaces. Charcoal is one of the best fuels available, its calorific value being so great; but its cost is in some places almost prohibitive. Wood burns too quickly, and is therefore expensive, and necessitates incessant firing.
For practical purposes we are thus often left to a selection of some type of coal. A coal with comparatively little heating power at a cheap price will be found more expensive in the end than one costing more, but capable of more rapid combustion and possessing more heat yielding gases. Cheap and hard coals give the fireman an amount of labor which is excessive. The proper maintenance of the temperature of the stove is almost impossible. Anthracite is excellent in every way, as it consists of nearly pure carbon, giving off a high degree of heat without smoke. Its use, of course, necessitates the use of a blower, but to this there can be no objection. Any coal which will burn freely and clean, giving off no excessive smoke, and capable of almost complete combustion, will give satisfaction in enameling; but it must not be forgotten that the consumption of fuel is so large that both price and quality must be carefully considered. Experimental tests must be made from time to time. A cheap, common coal will never give good results, and a good expensive coal will make the cost of manufacture so great that the prices of the enameled articles will render them unsalable. Any ordinary small factory will use from 2 to 4 tons per day of coal, and it will thus be seen that the financial success of a concern lies to a very great extent at the mouth of the furnace. Coke is a good medium for obtaining the necessary heat required in enameling if it can be got at a reasonable price. With a good draught a uniform temperature can be easily kept up, and the use of this by-product is, therefore, to be recommended.