 The
Science Notebook
The
Science NotebookHenley's Book of Formulas, Recipes and Processes
Home Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!
Henley's Twentieth Century Book of Formulas, Recipes and Processes - Pages 651-675
[651]
SOAPS
acid unites with the soda lye, forming a salt, which is regarded as soap. By the addition of sodium chloride, this (the soap) is separated and swims on the residual liquid as "kern," or granulated soap. Good soaps were formerly made only from animal fats, but some of the vegetable oils or fats have been found to also make excellent soap. Among them the best is cacao butter.
From a hygienic standpoint it must be accepted as a law that a good toilet soap must contain no free (uncombined) alkali, every particle of it must be chemically bound up with fatty acid to the condition of a salt, and the resultant soap should be neutral in reaction. Many of the soaps found in commerce to-day contain free alkali, and exert a harmful effect upon the skin of those who use them. Such soaps may readily be detected by bringing them into contact with the tongue. If free alkali be present it will make itself known by causing a burning sensation something that a good toilet soap should never do.
The efficiency of soap depends upon the fact that in the presence of an abundance of water the saponified fat is decomposed into acid and basic salts, in which the impurities of the skin are dissolved and are washed away by the further application of water. Good soap exerts its effects on the outer layer of the skin, the so-called horny (epithelial) layer, which in soapy water swells up and is, in fact, partially dissolved in the medium and washed away. This fact, however, is unimportant, since the superficial skin cells are reproduced with extraordinary rapidity and ease. When a soap contains or carries free alkali, the caustic effects of the latter are carried further and deeper, reaching below the epithelial cells and attacking the true skin, in which it causes minute rifts and splits and renders it sore and painful. Good soap, on the contrary, makes the skin smooth and soft.
Since the employment of poor soaps works so injuriously upon the skin, many persons never, or rarely ever, use soap, but wash the face in water alone, or with a little almond bran added. Their skins cannot bear the regular application of poor soap. This, however, applies only to poor, free-alkali containing soaps. Any skin can bear without injury any amount of a good toilet soap, free from uncombined alkali and other impurities. The habit of washing the face with water only, without the use of soap, must be regarded as one altogether bad, since the deposits on the skin, mostly dust particles and dead epithelial cells, mingling with the oily or greasy matter exuded from the fat glands of the skin excellent nutrient media for colonies of bacteria cannot be got rid of by water alone. Rubbing only forces the mass into the openings in the skin (the sweat glands, fat glands, etc.), and stops them up. In this way are produced the so called "black heads" and other spots and blotches on the skin usually referred to by the uneducated, or partially educated, as "parasites." The complexion is in this manner injured quite as much by the failure to use good soap as by the use of a poor or bad article.
All of the skin troubles referred to may be totally avoided by the daily use of a neutral, alkali free soap, and the complexion thus kept fresh and pure. Completely neutral soaps, however, are more difficult to manufacture requiring more skill and care than those in which no attention is paid to excess of alkali and consequently cost more than the general public are accustomed, or, in fact, care to pay for soaps. While this is true, one must not judge the quality of a soap by the price demanded for it. Some of the manufacturers of miserable soaps charge the public some of the most outrageous prices. Neither can a soap be judged by its odor or its style of package and putting on the market.
To give a soap an agreeable odor the manufacturers add to it, just when it commences to cool off, an etheric oil (such as attar of rose, oil of violets, bergamot oil, etc.), or some balsamic material (such as tincture of benzoin, for instance). It should be known, however, that while grateful to the olfactory nerves, these substances do not add one particle to the value of the soap, either as a detergent or as a preserver of the skin or complexion.
Especially harmful to the skin are soaps containing foreign substances, such, for instance, as the starches, gelatin, clay, chalk, gums, or rosins, potato flour, etc., which are generally added to increase the weight of soap. Such soaps are designated, very significantly, "filled soaps," and, as a class, are to be avoided, if for no other reason, on account of their lack of true soap content. The use of these fillers should be regarded as a criminal falsification under the laws regarding articles of domestic use, since they are sold at a relatively high price, yet contain foreign matter, harmful to health.
[652]
SOAPS
RECIPES FOR COLD-STIRRED TOILET SOAPS.
Parts by weight
I.
Cocoanut oil 30
Castor oil 3
Caustic soda lye (38º Be) 17 1/2
Pink Soap.
Parts by weight
II.
Pink No. 114 10
Lemon oil 60
Cedarwood oil 60
Citronella oil 50
Wintergreen oil 15
Pale-Yellow Soap.
Parts by weight
III.
Orange No. 410 10
Citronella oil 60
Sassafras oil 60
Lavender oil 45
Wintergreen oil 15
Aniseed oil 25
Toilet Soap Powder.
Marseilles soap, powdered 100 parts
Bran of almonds 50 parts
Lavender oil 5 parts
Thyme oil 3 parts
Spike oil 2 parts
Citronella oil 2 parts
Soft Toilet Soaps. Soft toilet soaps or creams may be prepared from fresh lard with a small addition of cocoanut oil and caustic potash solution, by the cold process or by boiling. For the cold process, 23 parts of fresh lard and 2 parts of Cochin cocoanut oil are warmed in a jacketed pan, and when the temperature reaches 113º F. are treated with 9 parts of caustic potash and 2 1/2 parts of caustic soda solution, both of 38º Bé. strength, the whole being stirred until saponification is complete. The soap is transferred to a large marble mortar and pounded along with the following scenting ingredients: 0.15 parts of oil of bitter almonds and 0.02 parts of oil of geranium rose, or 0.1 part of the latter, and 0.05 parts of lemon oil. The warm process is preferable, experience having shown that boiling is essential to the proper saponification of the fats. In this method, 80 parts of lard and 20 parts of Cochin cocoanut oil are melted together in a large pan, 100 parts of potash lye (20º Bé.) being then crutched in by degrees, and the mass raised to boiling point. The combined influence of the heat and crutching vaporizes part of the water in the lye, and the soap thickens. When the soap has combined, the fire is made up, and another 80 parts of the same potash lye are crutched in gradually. The soap gets thicker and thicker as the water is expelled and finally throws up "roses" on the surface, indicating that it is nearly finished. At this stage it must be crutched vigorously, to prevent scorching against the bottom of the pan and the resulting more or less dark coloration. The evaporation period may be shortened by using only 50 to 60 parts of lye at first, and fitting with lye of 25º to 30º strength. For working on the large scale iron pans heated by steam are used, a few makers employing silverlined vessels, which have the advantage that they are not attacked by the alkalI. Tinned copper pans are also useful. The process takes from 7 to 8 hours, and when the soap is finished it is transferred into stoneware vessels for storage. Clear vegetable oils (castor oil) may be used, but the soaps lack the requisite nacreous luster required.
TRANSPARENT SOAPS.
The mode of production is the same for all. The fats are melted together, sifted into a double boiler, and the lye is stirred in at 111º F. Cover up for an hour, steam being allowed to enter slowly. There is now a clear, grain-like soap in the kettle, into which the sugar solution and the alcohol are crutched, whereupon the kettle is covered up. If cuttings are to be used, they are now added. When same are melted, the kettle will contain a thin, clear soap, which is colored and scented as per directions, and subsequently filled into little iron molds and cooled.
Rose-Glycerine Soap.
I.
Cochin cocoanut oil 70,000 parts
Compressed tallow 40,000 parts
Castor oil 30,000 parts
Caustic soda lye, 38º Bé. 79,000 parts
Sugar 54,000 parts
Dissolved in
Water 60,000 parts
Alcohol 40,000 parts
Geranium oil (African) 250 parts
Lemon oil 200 parts
Palmarosa oil 1,200 parts
Bergamot oil 80 parts
Benzoin -Glycerine Soap.
II.
Cochin cocoanut oil 66,000 parts
Compressed tallow 31,000 parts
[653]
SOAPS
Castor oil 35,000 parts
Caustic soda lye, 38º Bé 66,000 parts
Sugar 35,000 parts
Dissolved in
Water 40,000 parts
Alcohol 35,000 parts
Brown, No. 120 200 parts
Powdered benzoin (Siam) 4,200 parts
Styrax liquid 1,750 parts
Tincture of benzoin 1,400 parts
Peru balsam 700 parts
Lemon oil 200 parts
Clove oil 70 parts
Sunflower -Glycerine Soap.
III.
Cochin cocoanut oil 70,000 parts
Compressed tallow 50,000 parts
Castor oil 23,000 parts
Caustic soda lye, 39º Bé. 71,000 parts
Sugar 40,000 parts
Dissolved in
Water 30,000 parts
Alcohol 40,000 parts
Brown, No. 55 250 parts
Geranium oil 720 parts
Bergamot oil 300 parts
Cedarwood oil 120 parts
Palmarosa oil 400 parts
Vanillin 10 parts
Tonka tincture 400 parts
MISCELLANEOUS FORMULAS:
Szegedin Soap. Tallow, 120 parts; palm kernel oil, 80 parts. Saponify well with about 200 parts of lye of 24º Bé. and add, with constant stirring, the following fillings in rotation, viz., potash solution, 20º Bé., 150 parts, and cooling salt solution 20º Bé., 380 parts.
Instrument Soap. A soap for cleaning surgical instruments, and other articles of polished steel, which have become specked with rust by exposure, is made by adding precipitated chalk to a strong solution of cyanide of potassium in water, until a cream-like paste is obtained. Add to this white castile soap in fine shavings, and rub the whole together in a mortar, until thoroughly incorporated. The article to be cleaned should be first immersed, if possible, in a solution of 1 part of cyanide of potash in 4 parts of water, and kept there until the surface dirt and rust disappears. It should then be polished with the soap, made as above directed.
Stain-Removing Soaps. These are prepared in two ways, either by making a special soap, or by mixing ordinary soap with special detergents. A good recipe is as follows:
I.
Ceylon cocoanut or palm seed oil 320 pounds
Caustic soda lye, 38º Bé. 160 pounds
Carbonate of potash, 20º Bé. 56 pounds
Oil of turpentine 9 pounds
Finely powdered kieselguhr 280 pounds
Brilliant green 2 pounds
The oil having been fused, the dye is mixed with some of it and stirred into the contents of the pan. The kieselguhr is then crutched in from a sieve, then the lye, and then the carbonate of potash. These liquids are poured in in a thin stream. When the soap begins to thicken, add the turpentine, mold, and cover up the molds.
II.
Rosin grain soap 1,000 pounds
Talc (made to a paste with
weak carbonate of potash) 100 pounds
Oil of turpentine 4 pounds
Benzine 3 pounds
Mix the talc and soap by heat, and when cool enough add the turpentine and benzine, and mold.
III.
Cocoanut oil 600 pounds
Tallow 400 pounds
Caustic soda lye 500 pounds
Fresh ox gall 200 pounds
Oil of turpentine 12 pounds
Ammonia (sp. gr., 0.91) 6 pounds
Benzine 5 pounds
Saponify by heat, cool, add the gall and the volatile liquids, and mold.
Soap Substitutes.
I.
Linseed oil 28 pounds
Sulphur 8 pounds
Aluminum soap 28 pounds
Oil of turpentine 4 pounds
II.
Aluminum soap 15 pounds
Almadina 25 pounds
Caoutchouc 50 pounds
Sulphur 6 pounds
Oleum succini 4 pounds
Shampoo Soap.
Linseed oil 20 parts
Malaga olive oil 20 parts
Caustic potash 9 1/2 parts
Alcohol 1 part
Water 30 parts
[654]
SOAPS
Warm the mixed oils on a large water bath, then the potash and water in another vessel, heating both to 158º F., and adding the latter hot solution to the hot oil while stirring briskly. Now add and thoroughly mix the alcohol. Stop stirring, keep the heat at 158º F. until the mass becomes clear and a small quantity dissolves in boiling water without globules of oil separating. Set aside for a few days before using to make the liquid soap.
The alcohol may be omitted if a transparent product is immaterial.
Sapo Durus.
Olive oil 100 parts
Soda lye, sp. gr., 1.33 50 parts
Alcohol (90 per cent) 30 parts
Heat on a steam bath until saponification is complete. The soap thus formed is dissolved in 300 parts of hot distilled water, and salted out by adding a filtered solution of 25 parts of sodium chloride and 5 parts of crystallized sodium carbonate in 80 parts of water.
Sapo Mollis.
Olive oil 100 parts
Solid potassium hydroxide 21 parts
Water 100 parts
Alcohol (90 per cent) 20 parts
Boil by means of a steam bath until the oil is saponified, adding, if necessary, a little more spirit to assist the saponification.
Sand Soap. Cocoa oil, 24 parts; soda lye, 38º Bé., 12 parts; sand, finely sifted, 28 parts; cassia oil, .0100 parts; sassafras oil, .0100 parts.
Salicylic Soap. When salicylic acid is used in soap it decomposes, as a rule, and an alkali salicylate is formed which the skin does not absorb. A German chemist claims to have overcome this defect by thoroughly eliminating all water from potash or soda soap, then mixing it with vaseline, heating the mixture, and incorporating free salicylic acid with the resulting mass. The absence of moisture prevents any decomposition of the salicylic acid.
Olein Soap Substitute. Fish oil or other animal oil is stirred up with sulphuric acid, and then treated with water. After another stirring, the whole is left to settle, and separate into layers, whereupon the acid and water are drawn off, and caustic soda solution is stirred in with the oil. The finishing stage consists in stirring in refined mineral oil, magnesium chloride, borium chloride, and pure seal or whale oil, in succession.
Mottled Soap. Tallow, 30 parts; palm kernel oil, 270 parts; lye, 20º, 347 1/2 parts; potassium chloride solution, 20, 37 1/2 parts. After everything has been boiled into a soap, crutch the following dye solution into it: Water, 5 parts; blue, red, or black, .0315 parts; water glass, 38º, 10 parts; and lye, 38º, 1 1/2 parts.
Laundry Soap. A good, common hard soap may be made from clean tallow or lard and caustic soda, without any very special skill in manipulation. The caustic soda indicated is a crude article which may now be obtained from wholesale druggists in quantities to suit, at a very moderate price. A lye of average strength is made by dissolving it in water in the proportion of about 2 pounds to the gallon. For the saponification of lard, a given quantity of the grease is melted at a low heat, and 1/4 its weight of lye is then added in small portions with constant stirring; when incorporation has been thoroughly effected, another portion of lye equal to the first is added, as before, and the mixture kept at a gentle heat until saponification appears to be complete. If the soap does not readily separate from the liquid, more lye should be added, the soap being insoluble in strong lye. When separation has occurred, pour off the lye, add water to the mass, heat until dissolved, and again separate by the use of more strong lye or a strong solution of common salt. The latter part of the process is designed to purify the soap and may be omitted where only a cruder article is required. The soap is finally remelted on a water bath, kept at a gentle heat until as much water as possible is expelled, and then poured into frames or molds to set.
Dog Soap.
Parts by weight
Petroleum 5
Wax 4
Alcohol 5
Good laundry soap 15
Heat the petroleum, wax, and alcohol on a water bath until they are well mixed, and dissolve in the mixture the soap cut in fine shavings. This may be used on man or beast for driving away vermin.
Liquid Tar Soap (Sapo Picis liquidus).
Wood tar 25 parts
Hebra's soap spirit 75 parts
Ox Gall Soap for Cleansing Silk Stuffs. To wash fine silk stuffs, such as
[655]
SOAPS - SOLDERS
piece goods, ribbons, etc., employ a soap containing a certain amount of ox gall, a product that is not surpassed for the purpose. In making this soap the following directions will be found of advantage: Heat 1 pound of cocoanut oil to 100º F. in a copper kettle. While stirring vigorously add 1/2 pound of caustic soda lye of 30º Baumé. In a separate vessel heat 1/2 pound of white Venice turpentine, and stir this in the soap in the copper kettle. Cover the kettle well, and let it stand, mildly warmed for 4 hours, when the temperature can be again raised until the mass is quite hot and flows clear; then add the pound of ox gall to it. Now pulverize some good, perfectly dry grain soap, and stir in as much of it as will make the contents of the copper kettle so hard that it will yield slightly to the pressure of the fingers. From 1 to 2 pounds is all the grain soap required for the above quantity of gall soap. When cooled, cut out the soap and shape into bars. This is an indispensable adjunct to the dyer and cleaner, as it will not injure the most delicate color.
SOAP BUBBLE LIQUIDS.
I.
White hard soap 25 parts
Glycerine 15 parts
Water 1,000 parts
II.
Dry castile soap 2 parts
Glycerine 30 parts
Water 40 parts
SOAP POLISHES:
See Polishes.
SOAP, TOOTH:
See Dentifrice.
SODA PAINT:
See Paint.
SODA WATER:
See Beverages.
SODIUM HYPOSULPHITE:
See Photography.
SODIUM SILICATE AS A CEMENT:
See Adhesives, under Water-Glass Cements.
SODIUM SALTS, EFFERVESCENT:
See Salts.
Solders
SOLDERING OF METALS AND THE PREPARATION OF SOLDERS.
The object of soldering is to unite two portions of the same metal or of different metals by means of a more fusible metal or metallic alloy, applied when melted, and known by the name of solder. As the strength of the soldering depends on the nature of the solder used, the degree of strength required for the joint must be kept in view in choosing a solder. The parts to be joined must be free from oxide and thoroughly clean; this can be secured by filing, scouring, scraping, or pickling with acids. The edges must fit exactly, and be heated to the melting point of the solder. The latter must have a lower melting point than either of the portions of metal that require to be joined, and if possible only those metals should be chosen for solder which form alloys with them. The solder should also as far as possible have the same color and approximately the same strength as the article whose edges are to be united.
To remove the layers of oxide which form during the process of soldering, various so called "fluxes" are employed. These fluxes are melted and applied to the joint, and act partly by keeping off the air, thus preventing oxidation, and partly by reducing and dissolving the oxides themselves. The choice of a flux depends on the quantity of heat required for soldering.
Solders are classed as soft and hard solders. Soft solders, also called tin solders or white solders, consist of soft, readily fusible metals or alloys, and do not possess much strength; they are easy to handle on account of their great fusibility. Tin, lead-tin, and alloys of tin, lead, and bismuth are used for soft solders, pure tin being employed only for articles made of the same metal (pure tin).
The addition of some lead makes the solder less fusible but cheaper, while that of bismuth lowers the melting point. Soft solders are used for soldering easily fusible metals such as Britannia metal, etc., also for soldering tin plate. To prepare solder, the metals are melted together in a graphite crucible at as low a temperature as possible, well stirred with an iron rod, and cast into ingots in an iron mold. To melt the solder when required for soldering, the soldering iron is used; the latter should be kept as free from oxidation as possible, and the part applied should be tinned over.
To make so-called "Sicker" solder, equal parts of lead and tin are melted together, well mixed, and allowed to stand till the mixture begins to set, the part still in a liquid condition being then poured off. This mixture can, however,
[656]
SOLDERS
be more easily made by melting together 37 parts of lead and 63 parts of tin (exactly measured).
Soldering irons are usually made of copper, as copper is easily heated and easily gives up its heat to the solder. The point of the iron must be "tinned." To do this properly, the iron should be heated hot enough easily to melt the solder; the point should then be quickly dressed with a smooth flat file to remove the oxide, and rubbed on a piece of tin through solder and sal ammoniac. The latter causes the solder to adhere in a thin, even coat to the point of the iron. A gas or gasoline blow torch or a charcoal furnace is best for heating the iron, but a good, clean coal fire, well coked, will answer the purpose.
When in use, the iron should be hot enough to melt the solder readily. A cold iron produces rough work. This is where the beginner usually fails. If possible, it is well to warm the pieces before applying the iron. The iron must not be heated too hot, however, or the tin on the point will be oxidized. The surfaces to be soldered must be clean. Polish them with sandpaper, emery cloth, a file, or a scraper. Grease or oil will prevent solder from sticking.
Some good soldering fluid should be used. A very good fluid is made by dissolving granulated zinc in muriatic acid. Dissolve as much zinc as possible in the acid. The gas given off will explode if ignited. To granulate the zinc, melt it in a ladle, and pour it slowly into a barrel of water. A brush or swab should be used to spread the fluid on the surfaces to be soldered. If the point of the soldering iron becomes dirty, it should be wiped on a cloth or piece of waste that has been dampened with the soldering fluid.
Soldering of Metallic Articles. In a recently invented process the parts to be united are covered, on the surfaces not to be soldered, with a protective mass, which prevents an immediate contact of the solder with the surfaces in question, and must be brushed off only after the soldered pieces have cooled perfectly, whereby the possibility of a change of position of these pieces seems precluded.
For the execution of this process the objects to be soldered, after the surfaces to be united have been provided with a water glass solution as the soldering agent and placed together as closely as possible or united by wires or rivets, are coated in the places where no solder is desired with a protective mass, consisting essentially of carbon (graphite, coke, or charcoal), powdered talc or asbestos, ferric hydrate (with or without ferrous hydrate), and, if desired, a little aluminum oxide, together with a binding agent of the customary kind (glue solution, beer).
Following are some examples of the composition of these preparations:
I. Graphite, 50 parts; powdered coke, 5 parts; powdered charcoal, 5 parts; powdered talc, 10 parts; glue solution, 2.5 parts; drop beer, 2.5 parts; ferric hydrate, 10 parts; aluminum oxide, 5 parts.
II. Graphite, burnt, 4 parts; graphite, unburnt, 6 parts; powdered charcoal, 3 parts; powdered asbestos, 1 part; ferric hydrate, 3 parts; ferrous hydrate, 2 parts; glue solution, 1 part.
The article thus prepared is plunged, after the drying of the protective layer applied, in the metal bath serving as solder (molten brass, copper, etc.), and left to remain therein until the part to be soldered has become red hot, which generally requires about 50 to 60 seconds, according to the size of the object. In order to avoid, in introducing the article into the metal bath, the scattering of the molten metal, it is well previously to warm the article and to dip it warm. After withdrawal from the metal bath the soldered articles are allowed to cool, and are cleaned with wire brushes, so as to cause the bright surfaces to reappear.
The process is especially useful for uniting iron or steel parts, such as machinery, arms, and bicycle parts in a durable manner.
Soldering Acid. A very satisfactory soldering acid may be made by the use of the ordinary soldering acid for the base and introducing a certain proportion of chloride of tin and sal ammoniac. This gives an acid which is superior in every way to the old form. To make 1 gallon of this soldering fluid take 3 quarts of common muriatic acid and allow it to dissolve as much zinc as it will take up. This method, of course, is the usual one followed in the manufacture of ordinary soldering acid. The acid, as is well known, must be placed in an earthenware or glass vessel. The zinc may be sheet clippings or common plate spelter broken into small pieces. Place the acid in the vessel and add the zinc in small portions so as to prevent the whole from boiling over. When all the zinc has been added and the action has stopped, it indicates that enough has been taken up. Care must be taken to see that there is a little zinc left in the bottom, as other
[657]
SOLDERS
wise the acid will be in excess. The idea is to have the acid take up as much zinc as it can.
After this has been done there will remain some residue in the form of a black precipitate. This is the lead which all zinc contains, and which is not dissolved by the muriatic acid. This lead may be removed by filtering through a funnel in the bottom of which there is a little absorbent cotton, or the solution may be allowed to remain overnight until the lead has settled and the clear solution can then be poured off. This lead precipitate is not particularly injurious to the soldering fluid, but it is better to get rid of it so that a good, clear solution may be obtained. Next, dissolve 6 ounces of sal ammoniac in a pint of warm water. In another pint dissolve 4 ounces of chloride of tin. The chloride of tin solution will usually be cloudy, but this will not matter. Now mix the 3 solutions together. The solution will be slightly cloudy when the 3 have been mixed, and the addition of a few drops of muriatic acid will render it perfectly clear. Do not add any more acid than is necessary to do this, as the solution would then contain too much of this ingredient and the results would be injurious.
This soldering acid will not spatter when the iron is applied to it. It has also been found that a poorer grade of solder may be used with it than with the usual soldering acid.
ALUMINUM SOLDERS.
To solder aluminum it is necessary previously to tin the parts to be soldered. This tinning is done with the iron, using a composition of aluminum and tin. Replace the ordinary soldering iron by an iron of pure aluminum. Preparation of aluminum solder: Commence by fusing the copper; then add the aluminum in several installments, stir the mixture well with a piece of iron; next add the zinc and a little tallow or benzine at the same time. Once the zinc is added do not heat too strongly, to avoid the volatilization of the zinc.
I. Take 5 parts of tin and 1 part of aluminum. Solder with the iron or with the blowpipe, according to the article in question.
II. The pieces to be soldered are to be tinned, but instead of using pure tin, alloys of tin with other metals are employed, preferably those of tin and aluminum. For articles to be worked after soldering, 45 parts of tin and 10 parts of aluminum afford a good alloy, malleable enough to be hammered, cut, or turned. If they are not to be worked, the alloy requires less aluminum and may be applied in the usual manner as in soldering iron.
Aluminum Bronze.
I. Strong solder: Gold, 89 parts; fine silver, 5 parts; copper, 6 parts.
II. Medium solder: Gold, 54 parts; fine silver, 27 parts; copper, 19 parts.
III. Weak solder: Gold, 14 parts; silver, 57 parts; copper, 15 parts; brass, 14 parts.
BRASS SOLDERS.
Brass solder consists of brass fusible at a low temperature, and is made by melting together copper and zinc, the latter being in excess. A small quantity of tin is often added to render the solder more fusible. Hard solders are usually sold in the form of granules. Although many workers in metals make their own solder, it is advisable to use hard solder made in factories, as complete uniformity of quality is more easily secured where large quantities are manufactured.
In making hard solder the melted metal is poured through birch twigs in order to granulate it. The granules are afterwards sorted by passing them through sieves.
When brass articles are soft-soldered, the white color of the solder contrasts unpleasantly with the brass. If this is objected to, the soldered part can be colored yellow in the following manner:
Dissolve 10 parts of copper sulphate in 35 parts of water; apply the solution to the solder, and stir with a clean iron wire. This gives the part the appearance of copper. To produce the yellow color, paint the part with a mixture consisting of 1 part of a solution of equal parts of zinc and water (1 part each) and 2 parts of a solution of 10 to 35 parts respectively of copper sulphate and water and rub on with a zinc rod. The resulting yellow color can, if desired, be improved by careful polishing.
The quality of soft solder is always judged in the trade from the appearance of the surface of the castings, and it is considered important that this surface should be radiant and crystalline, showing the so-called "flowers." These should be more brilliant than the dull background, the latter being like mat silver in appearance. If the casting has a uniform whitish-gray color, this is an indication that the alloy contains an insufficient quantity of tin. In this case
[658]
SOLDERS
the alloy should be remelted and tin added, solder too poor in tin being extremely viscid.
Most of the varieties of brass used in the arts are composed of from 68 to 70 per cent copper and from 32 to 30 per cent zinc. Furthermore, there are some kinds of brass which contain from 24 to 40 per cent zinc. The greater the quantity of zinc the greater will be the resemblance of the alloy to copper. Consequently, the more crystalline will the structure become. For hard soldering only alloys can be employed which, as a general rule, contain no more than 34 per cent of zinc. With an increase in copper there follows a rise in the melting point of the brass. An alloy containing 90 per cent of copper will meet at 1,940º F.; 80 per cent copper, at 1,868º F.; 70 per cent copper, at 1,796º F.; 60 per cent copper, at 1,742º F. Because an increase in zinc causes a change in color, it is sometimes advisable to use tin for zinc, at least in part, so that the alloy becomes more bronze-like in its properties. The durability of the solder is not seriously affected, but its fusibility is lowered. If more than a certain proportion of tin be added, thin and very fluid solders are obtained of grayish-white color, and very brittle indeed, so brittle that the soldering joints are apt to open if the object is bent. Because too great an addition of tin is injurious, the utmost caution must be exercised. If very refractory metals are to be soldered, brass alone can be used. In some cases, a solder can be produced merely by melting brass and adding copper. The following hard solders have been practically tested and found of value.
YELLOW HARD SOLDERS:
Applebaum's Compositions.
I.
Copper 58 parts
Zinc 42 parts
II.
Sheet brass 85.42 parts
Zinc 13.58 parts
Karmarsch's Composition.
III.
Brass 7 parts
Zinc 1 part
IV.
Zinc 49 parts
Copper 44 parts
Tin 4 parts
Lead 2 parts
Prechtl's Composition.
V.
Copper 53.3 parts
Zinc 43.1 parts
Tin 1.3 parts
Lead 0.3 parts
All these hard-solder compositions have the fine yellow color of brass, are very hard, and can be fused only at high temperatures. They are well adapted for all kinds of iron, steel, copper, and bronze.
Solders which fuse at somewhat lower temperatures and, therefore, well adapted for the working of brass, are the following:
VI.
Sheet brass 81.12 parts
Zinc 18.88 parts
VII.
Copper 54.08 parts
Zinc 45.29 parts
VIII.
Brass 3 to 4 parts
Zinc 1 part
A solder which is valuable because it can be wrought with the hammer, rolled put, or drawn into wire, and because it is tough and ductile, is the following:
IX.
Brass 78.26 parts
Zinc 17.41 parts
Silver 4.33 parts
Fusible White Solder.
X.
Copper 57.4 parts
Zinc 28 parts
Tin 14.6 parts
Easily Fusible Solders.
XI.
Brass 5 parts
Zinc 2.5 parts
XII.
Brass 5 parts
Zinc 5 parts
Semi-White Hard Solders.
XIII.
Copper 53.3 parts
Zinc 46.7 parts
XIV.
Brass 12 parts
Zinc 4 to 7 parts
Tin 1 part
XV.
Brass 22 parts
Zinc 10 parts
Tin 1 part
XVI.
Copper 44 parts
Zinc 49 parts
Tin 3.20 parts
Lead 1.20 parts
Formulas XIII and XVI are fairly fusible.
White Hard Solders.
XVII.
Brass 20 parts
Zinc 1 part
Tin 4 parts
XVIII.
Copper 58 parts
Zinc1 7 parts
Tin 15 parts
XIX.
Brass 11 parts
Zinc 1 part
Tin 2 parts
[659]
SOLDERS
XX.
Brass 6 parts
Zinc 4 parts
Tin 10 parts
XXI.
Copper 57.44 parts
Zinc 27.98 parts
Tin 14.58 parts
For Brass Tubes.
I. Copper, 100 parts; lead, 25 parts.
II. A very strong solder for soldering brass tubes to be drawn, etc., is composed of 18 parts brass, 4 parts zinc, and 1 part fine silver.
For Fastening Brass to Tin. To 20 parts of fine, reduced copper, add sufficient sulphuric acid to make a stiff paste. To this add 70 parts of metallic mercury, and work in, at the same time applying heat until the mass assumes a wax like consistency. Warm or heat the plates to be united, to about the same temperature, apply the mixture, hot, to each, then press together, and let cool.
COPPER SOLDERS.
The copper solders which are used for soldering copper as well as bronze are mixtures of copper and lead. By increasing the quantity of lead the fusibility is increased, but the mixture departs from the color and toughness of copper. The most commonly employed copper solder is the following:
I.
Copper 5 parts
Lead 1 part
II.
Copper 80 parts
Lead 15 parts
Tin 5 parts
For Red Copper.
I. Copper, 3 parts; zinc, 1 part.
II. Copper, 7 parts; zinc, 3 parts; tin, 2 parts.
FATS FOR SOLDERING.
I. Soldering fat or grease is commonly a mixture of rosin and tallow with the addition of a small quantity of sal ammoniac. It is particularly adapted to the soldering of tinned ware, because it is easily wiped off the surface after the joint is made, whereas if rosin were used alone, the scraping away might remove some of the tin and spoil the object.
II. The following is a well tried recipe for a soldering grease: In a pot of sufficient size and over a slow fire melt together 500 parts of olive oil and 400 parts of tallow; then stir in slowly 250 parts of rosin in powder, and let the whole boil up once. Now let it cool down, and add 125 parts of saturated solution of sal ammoniac, stirring the while. When cold, this preparation will be ready for use.
FLUIDS FOR SOLDERING.
I. To the ordinary zinc chloride, prepared by digesting chips of zinc in strong hydrochloric acid to saturation, add 1/3 spirits of sal ammoniac and 1/3 part rain water, and filter the mixture. This soldering liquid is especially adapted to the soft soldering of iron and steel, because it does not make rust spots.
To solder zinc, the zinc chloride may be used without any spirit sal ammoniac.
II. Mix phosphoric acid with strong spirits of wine in the following proportions:
Phosphoric acid solution 1 quart
Spirits of wine (80 per cent) 1 1/2 quarts
More or less of the spirits of wine is used depending upon the concentration of the phosphoric acid solution. When this soldering liquid is applied to the metal to be soldered, the phosphoric acid immediately dissolves the oxide. The hot soldering iron vaporizes the spirits of wine very quickly and causes the oxide released by the phosphoric acid to form a glazed mass with the surplus phosphoric acid, which mass can be easily removed.
III. Dissolve in hydrochloric acid: Zinc, 50 parts (by weight); sal ammoniac, 50 parts.
IV. Hydrochloric acid, 600 parts (by weight); sal ammoniac, 100 parts. Put zinc chips into the acid to saturation, next add the sal ammoniac. Filter when dissolved and preserve in flasks.
V. Eight hundred parts of water with 100 parts of lactic acid and 100 parts of glycerine. This dispenses with the use of chloride of zinc.
Acid Free Soldering Fluid.
I. Five parts of zinc chloride dissolved in 25 parts of boiling water. Or, 20 parts of zinc chloride, 10 parts of ammonia chloride, dissolved in 100 parts of boiling water and put into glass carboys.
II.
Chloride zinc 1 drachm
Alcohol 1 ounce
Substitute for Soldering Fluid. As a substitute for the customary soldering fluid and soldering mediums an ammonia soap is recommended, which is obtained by the mixture of a finely powdered rosin with strong ammonia solution. Of this soap only the finely divided
[660]
SOLDERS
rosin remains on the soldered place after the soldering. This soldering process is well adapted for soldering together copper wires for electrical conduits, since the rosin at the same time serves as an insulator.
FLUXES FOR SOLDERING.
The fluxes generally used in the softsoldering of metals are powdered rosin or a solution of chloride of zinc, alone or combined with sal ammoniac. A neutral soldering liquid can be prepared by mixing 27 parts neutral zinc chloride, 11 parts sal ammoniac, and 62 parts water; or, 1 part sugar of milk, 1 part glycerine, and 8 parts water.
A soldering fat for tin-plate, preferable to ordinary rosin, as it can be more easily removed after soldering, is prepared as follows: One hundred and fifty parts beef tallow, 250 parts rosin, and 150 parts olive oil are melted together in a crucible and well stirred, 50 parts powdered sal ammoniac dissolved in as little water as possible being added.
Soldering fat for iron is composed of 50 parts olive oil and 50 parts powdered sal ammoniac. Soldering fat for aluminum is made by melting together equal parts of rosin and tallow, half the quantity of zinc chloride being added to trie mixture.
Soldering paste consists of neutral soldering liquid thickened with starch paste. This paste must be applied more lightly than the soldering liquid.
Soldering salt is prepared by mixing equal parts of neutral zinc chloride, free from iron, and powdered sal ammoniac. When required for use, 1 part of the salt should be dissolved in 3 or 4 parts water.
Borax is the flux most frequently used for hard-soldering; it should be applied to the soldering seam either dry or stirred to a paste with water. It is advisable to use calcined borax, i.e, borax from which the water of crystallization has been driven out by heat, as it does not become so inflated as ordinary borax. Borax dissolves the metallic oxides forming on the joint.
Finely powdered cryolite, or a mixture of 2 parts powdered cryolite and 1 part phosphoric acid, is also used for hard soldering copper and copper alloys.
Muller's hard-soldering liquid consists of equal parts of phosphoric acid and alcohol (80 per cent)
A mixture of equal parts of cryolite and barium chloride is used as a flux in hard-soldering aluminum bronze.
A very good dry-soldering preparation consists of two vials, one of which is filled with zinc chloride, and the other with ammonium chloride. To use, dissolve a little of each salt in water, apply the ammonium chloride to the object to be soldered and heat the latter until it begins to give off vapor of ammonium, then apply the other, and immediately thereafter the solder, maintaining the heat in the meantime. This answers for very soft solder. For a harder solder dissolve the zinc in a very small portion of the ammonium chloride solution (from 1/4 to 1/2 pint).
When steel is to be soldered on steel, or iron on steel, it is necessary to remove every trace of oxide of iron between the surfaces in contact. Melt in an earthen vessel: Borax, 3 parts; colophony, 2 parts; pulverized glass, 3 parts; steel filings, 2 parts; carbonate of potash, 1 part; hard soap, powdered, 1 part. Flow the melted mass on a cold plate of sheet iron, and after cooling break up the pieces and pulverize them. This powder is thrown on the surfaces a few minutes before the pieces to be soldered are brought together. The borax and glass contained in the composition dissolve, and consequently liquefy all of the impurities, which, if they were shut up between the pieces soldered, might form scales, at times dangerous, or interfering with the resistance of the piece.
To prepare rosin for soldering bright tin, mix 1 1/2 pounds of olive oil, 1 1/2 pounds of tallow, and 12 ounces of pulverized rosin, and let them boil up. When this mixture has become cool, add 1 3/8 pints of water saturated with pulverized sal ammoniac, stirring constantly
GAS SOLDERING.
The soldering of small metallic articles where the production is a wholesale one, is almost exclusively done by the use of gas, a pointed flame being produced by air pressure. The air pressure is obtained by the workman who does the soldering setting in motion a treadle with his foot, which, resting on rubber bellows, drives by pressure on the same the aspirated air into wind bellows. From here it is sent into the soldering pipe, where it is connected with the gas and a pointed flame is produced. In order to obtain a rather uniform heat the workman has to tread continually, which, however, renders it almost impossible to hold the article to be soldered steady, although this is necessary if the work is to proceed quickly. Hence, absolutely skillful and expensive hands are required, on whom the employer is often entirely dependent. To improve
[661]
SOLDERS
this method of soldering and obviate its drawbacks, the soldering may be conducted with good success in the following manner: For the production of the air current a small ventilator is set up. The wind is conducted through two main conduits to the work tables. Four or six tables may, for instance, be placed together, the wind and the gas pipe ending in the center. The gas is admitted as formerly, the wind is conducted into wind bellows by means of joint and hose to obtain a constant pressure and from here into the soldering pipe. In this manner any desired flame may be produced, the workman operates quietly and without exertion, which admits of employing youthful hands and consequently of a saving in wages. The equipment is considerably cheaper, since the rubber bellows under the treadle are done away with.
GERMAN-SILVER SOLDERS.
Because of its peculiar composition German-silver solder is related to the ordinary hard solders. Just as hard solders may be regarded as varieties of brass to which zinc has been added, German-silver solders may be regarded as German silver to which zinc has been added. The German-silver solder becomes more easily fused with an increase in zinc, and vice versa. If the quantity of zinc be increased beyond a certain proportion, the resultant solder becomes too brittle. German-silver solders are characterized by remarkable strength, and are therefore used not only in soldering German silver, but in many cases where special strength is required. As German silver can be made of the color of steel, it is frequently used for soldering fine steel articles.
Solder for ordinary German silver can be made of 1,000 parts German-silver chips, 125 parts sheet-brass chips, 142 parts zinc, and 33 parts tin; or, of 8 parts German silver and 2 to 3 parts zinc.
Soft German-Silver Solder.
I.
Copper 4.5 parts
Zinc 7 parts
Nickel 1 part
II.
Copper 35 parts
Zinc 56.5 parts
Nickel 8.5 parts
III.
German silver 5 parts
Zinc 5 parts
Compositions I and II have analogous properties. In composition III German silver" is to be considered as a mixture of copper, zinc, and nickel, for which reason it is necessary to know the exact composition of the German silver to be used. Otherwise it is advisable to experiment first with small quantities in order to ascertain how much zinc is to be added. The proper proportion of German silver to zinc is reached when the mixture reveals a brilliancy and condition which renders it possible to barely pulverize it while hot. A small quantity when brought in contact with the soldering iron should just fuse.
Hard German-Silver or Steel Solder.
I.
Copper 35 parts
Zinc 56.5 parts
Nickel 9.5 parts
II.
Copper 38 parts
Zinc 50 parts
Nickel 12 parts
Composition I requires a fairly high temperature in order to be melted. Composition II requires a blow pipe.
GOLD SOLDERS:
Hard Solder for Gold. The hard solder or gold solder which the jeweler frequently requires for the execution of various works, not only serves for soldering gold ware, but is also often employed for soldering fine steel goods, such as spectacles, etc. Fine gold is only used for soldering articles of platinum. The stronger the alloy of the gold, the more fusible must be the solder. Generally the gold solder is a composition of gold, silver, and copper. If it is to be very easily fusible, a little zinc may be added, but, on the other hand, even the copper is sometimes left out and a mixture consisting only of gold and silver (e. g., equal parts of both) is used. The shade of the solder also requires attention, which must be regulated by varying proportions of silver and copper, so that it may be as nearly as possible the same as that of the gold to be soldered.
I. For 24-carat gold: Twenty-two parts gold (24 carat), 2 parts silver, and 1 part copper; refractory.
II. For 18-carat gold: Nine parts gold (18 carat), 2 parts silver, and 1 part copper; refractory.
III. For 16-carat gold: Twenty-four parts gold (16 carat), 10 parts silver, and 8 parts copper; refractory.
IV. For 14-carat gold: Three parts gold (14 carat), 2 parts silver, and 1 part copper; more fusible.
V. Gold solder for alloys containing smaller quantities of gold is composed
[662]
SOLDERS
of 8 parts gold, 10.5 parts silver, and 5.5 parts copper, or,
VI. Ten parts gold (13.5 carat), 5 parts silver, and 1 part zinc.
VII. The following easily fusible solder is used for ordinary gold articles: Two parts gold, 9 parts silver, 1 part copper, and 1 part zinc. Articles soldered with this solder cannot be subjected to the usual process of coloring the gold, as the solder would become black.
VIII. A refractory enamel solder for articles made of 20-carat and finer gold, which can bear the high temperature required in enameling, consists of 37 parts gold and 9 parts silver, or 16 parts gold (18 carat), 3 parts silver, and 1 part copper.
Which of these compositions should be employed depends upon the degree of the fusibility of the enamel to be applied. If it is very difficult of fusion only the first named can be used; otherwise it may happen that during the melting on of the enamel the soldering spots are so strongly heated that the solder itself melts. For ordinary articles, as a rule, only readily fusible enamels are employed, and consequently the readily fusible enameling solder may here be made use of. Soldering with the latter is readily accomplished with the aid of the soldering pipe. Although the more hardly fusible gold solders may also be melted by the use of the ordinary soldering pipe, the employment of a special small blowing apparatus is recommended on account of the resulting ease and rapidity of the work.
SOLDERS FOR GLASS.
I. Melt tin, and add to the melted mass enough copper, with constant stirring, until the melted metal consists of 95 per cent of tin and 5 per cent of copper. In order to render the mixture more or less hard, add 1/2 to 1 per cent of zinc or lead.
II. A compound of tin (95 parts) and zinc (5 parts) melts at 392º F., and can then be firmly united to glass. An alloy of 90 parts of tin and 10 parts of aluminum melts at 734º F., adheres, like the preceding, to glass, and is equally brilliant. With either of these alloys glass may be soldered as easily as metal, in two ways. In one, heat the pieces of glass in a furnace and rub a stick of soldering alloy over their surfaces. The alloy will melt, and can be easily spread by means of a roll of paper or a slip of aluminum. Press the pieces firmly together, and keep so until cool. In the other method a common soldering iron, or a rod of aluminum, is heated over a coal fire, a gas jet, or a flame supplied by petroleum. The hot iron is passed over the alloy and then over the pieces to be soldered, without the use of a dissolvent. Care should be taken that neither the soldering irons nor the glass be brought to a temperature above the melting point of the alloy, lest the latter should be oxidized, and prevented from adhering.
HARD SOLDERS.
Hard solders are distinguished as brass, German silver, copper, gold, silver, etc., according to the alloys used (see Brass Solders, Copper Solders, etc., for other hard solders).
The designation "hard solder" is used to distinguish it from the easily running and softer solder used by tinsmiths, and it applies solely to a composition that will not flow under a red heat. For the purposes of the jeweler solder may be classified according to its composition and purpose, into gold or silver solder, which means a solder consisting of an alloy of gold with silver, copper, tin, or zinc like metal or an alloy of silver with copper, tin, or zinc like metal. According to the uses, the solder is made hard or soft; thus in gold solders there is added a greater amount of silver, whereas for silver solders there is added more tin or zinc like metal.
In the production of solder for the enameler's use, that is for combining gold with gold, gold with silver, or gold with copper, which must be enameled afterwards, it is necessary always to keep in mind that no solder can be used effectually that contains any tin, zinc, zinc alloys, or tin or zinc like metals in any great quantities, since it is these very metals that contribute to the cracking of the enamel. Yet it is not possible to do without such an addition entirely, otherwise the solder would not flow under the melting point of the precious metals themselves and we should be unable to effect a union of the parts. It is therefore absolutely necessary to confine these additions to the lowest possible percentage, so that only a trace is apparent. Moreover, care must be taken to use for enameling purposes no base alloy, because the tenacity or durability of the compound will be affected thereby; in other words, it must come up to the standard.
In hard soldering with borax, direct, several obstacles are encountered that make the process somewhat difficult. In
[663]
SOLDERS
the first place the salt forms great bubbles in contact with the soldering iron, and easily scales away from the surface of the parts to be soldered. Besides this, the parts must be carefully cleaned each time prior to applying the salt. All these difficulties vanish if instead of borax we use its component parts, boric acid and sodium carbonate. The heat of the soldering iron acting on these causes them to combine in such a way as to produce an excellent flux, free from the difficulties mentioned.
Composition of Various Hard Solders.
Yellow solders for brass, bronze, copper, and iron:
I. Sheet-brass chips, 5 parts, and zinc, 3 to 5 parts, easily fusible.
II. Sheet brass chips, 3 parts, and zinc, 1 part; refractory.
III. Sheet-brass chips, 7 parts, and zinc, 1 part; very refractory and firm.
Semi-white solders, containing tin and consequently harder:
I. Sheet brass, 12 parts; zinc, 4 to 7 parts, and tin, 1 part.
II. Copper, 16 parts; zinc, 16 parts, and tin, 1 part.
III. Yellow solder, 20 to 30 parts, and tin, 1 part.
White solders:
I. Sheet brass, 20 parts; zinc, 1 part, and tin, 4 parts.
II. Copper, 3 parts; zinc, 1 part, and tin, 1 part.
To Hard-Solder Parts Formerly Soldered with Tin Solder. To repair gold or silver articles which have been spoiled with tin solder proceed as follows: Heating the object carefully by means a of small spirit lamp, brush the tin off as much as possible with a chalk brush; place the article in a diluted solution of hydrochloric acid for about 8 to 10 hours, as required. If much tin remains, perhaps 12 hours may be necessary. Next withdraw it, rinse off and dry; whereupon it is carefully annealed and finally put in a pickle of dilute sulphuric acid, to remove the annealing film. When the article has been dipped, it may be hard soldered again.
SILVER SOLDERS.
Silver solder is cast in the form of ingots, which are hammered or rolled into thin sheets. From these small chips or "links," as they are called, are cut off. The melted solder can also be poured, when slightly cooled, into a dry iron mortar and pulverized while still warm. The solder can also be filed and the filings used for soldering.
Silver solders are used not only for soldering silver objects, but also for soldering metals of which great resistance is expected. A distinction must be drawn between silver solder consisting either of copper and silver alone, and silver solder to which tin has been added.
Very Hard Silver Solder for Fine Silverware.
I.
Copper 1 part
Silver 4 parts
Hard silver solder.
II.
Copper 1 part
Silver 20 parts
Brass 9 parts
III.
Copper 2 parts
Silver 28 parts
Brass 10 parts
Soft silver solder.
IV.
Silver 2 parts
Brass 1 part
V.
Silver 3 parts
Copper 2 parts
Zinc 1 part
VI.
Silver 10 parts
Brass 10 parts
Tin 1 part
These solders are preferably to be employed for the completion of work begun with hard silver solders, defective parts alone being treated. For this purpose it is sometimes advisable to use copper silver alloys mixed with zinc, as for example:
VII.
Silver 12 parts
Copper 4 parts
Zinc 1 part
VIII.
Silver 5 parts
Brass 6 parts
Zinc 2 parts
This last formula (VIII) is most commonly used for ordinary silverware.
Silver Solders for Soldering Iron, Steel, Cast Iron, and Copper.
I.
Silver 10 parts
Brass 10 parts
II.
Silver 20 parts
Copper 30 parts
Zinc 10 parts
III.
Silver 30 parts
Copper 10 parts
Tin 0.5 parts
IV.
Silver 60 parts
Brass 60 parts
Zinc 5 parts
[664]
SOLDERS
In those solders in which brass is used care should be taken that none of the metals employed contains iron. Even an inappreciable amount of iron deleteriously affects the solder.
V. Copper, 30 parts; zinc, 12.85 parts; silver, 57.15 parts.
VI. Copper, 23.33 parts; zinc, 10 parts; silver, 66.67 parts.
VII. Copper, 26.66 parts; zinc, 10 parts; silver, 63.34 parts.
VIII. Silver, 66 parts; copper, 24 parts, and zinc, 10 parts. This very strong solder is frequently used for soldering silver articles, but can also be used for soldering other metals, such as brass, copper, iron, steel band-saw blades, etc.
IX. Silver, 4 parts, and brass, 3 parts.
X. A very refractory silver solder, which, unlike the silver solder containing zinc, is of great ductility and does not break when hammered, is composed of 3 parts silver and 1 part copper.
Soft Silver Solders. I. A soft silver solder for resoldering parts already soldered is made of silver, 3 parts; copper, 2 parts, and zinc, 1 part.
II. Silver, 1 part, and brass, 1 part; or, silver, 7 parts; copper, 3 parts, and zinc, 2 parts.
III. A readily fusible silver solder for ordinary work: Silver, 5 parts; copper, 6 parts, and zinc, 2 parts.
IV. (Soft.) Copper, 14.75 parts; zinc, 8.20 parts; silver, 77.05 parts.
V. Copper, 22.34 parts; zinc, 10.48 parts; silver, 67.18 parts.
VI. Tin, 63 parts; lead, 37 parts.
French Solders for Silver.
I. For fine silver work: Fine silver, 87 parts; brass, 13 parts.
II. For work 792 fine: Fine silver, 83 parts; brass, 17 parts.
III. For work 712 fine: Fine silver, 75 parts; brass, 25 parts.
IV. For work 633 fine: Fine silver, 66 parts; brass, 34 parts.
V. For work 572 fine: Fine silver, 55 parts; brass, 45 parts.
Solder for Silversmiths, etc. Gold, 10 parts; silver, 55 parts; copper, 29 parts; zinc, 6 parts.
Hard Solder. Silver, 60 parts; bronze, 39 parts; arsenic, 1 part.
Soft Solder. Powdered copper, 30 parts; sulphate of zinc, 10 parts; mercury, 60 parts; sulphuric acid. Put the copper and the zinc sulphate in a porcelain mortar, and then the sulphuric acid. Enough acid is required to cover the composition; next add the mercury while stirring constantly. When the amalgamation is effected, wash several times with hot water to remove the acid, then allow to cool. For use, it is sufficient to heat the amalgam until it takes the consistency of wax. Apply on the parts to be soldered and let cool.
Solder for Silver Plated Work.
I. Fine silver, 2 parts; bronze, 1 part.
II. Silver, 68 parts; copper, 24 parts; zinc, 17 parts.
Solder for Silver Chains.
I. Fine silver, 74 parts; copper, 24 parts; orpiment, 2 parts.
II. Fine silver, 40 parts; orpiment, 20 parts; copper, 40 parts.
SOFT SOLDERS:
See also Brass Solders, Copper Solders, Gold Solders.
I. Fifty parts bismuth, 25 parts tin, and 25 parts lead. This mixture melts at 392º F.
II. Fifty parts bismuth, 30 parts lead, and 20 parts tin. This will melt at 374º F.
III. The solder that is used in soldering Britannia metal and block tin pipes is composed of 2 parts tin and 1 part lead. This melts in the blow-pipe flame at many degrees lower temperature than either tin or Britannia metal, and it is nearly of the same color. Care must be taken in mixing these solders to keep them well stirred when pouring into molds. Care should also be taken that the metal which melts at a higher temperature be melted first and then allowed to cool to the melting temperature of the next metal to be added, and so on. Articles to be soldered with these solders should be joined with a blow pipe to get the best results, but if a copper is used it must be drawn out to a long, thin point. For a flux use powdered rosin or sweet oil.
Tin solders for soldering lead, zinc, tin, tin-plate, also copper and brass when special strength is not required, are prepared as follows:
I. Tin, 10 parts; lead, 4 parts; melting point, 356º F.
II. Tin, 10 parts; lead, 5 parts; melting point, 365º F.
III. Tin, 10 parts; lead, 6 parts; melting point, 374º F.
[665]
SOLDERS
IV. Tin, 10 parts; lead, 10 parts; melting point, 392º F.
V. Tin, 10 parts; lead, 15 parts; melting point, 432º F.
VI. Tin, 10 parts; lead, 20 parts; melting point, 464º F.
The last of the above mixtures is the cheapest, on account of the large quantity of lead.
Bismuth solder or pewterer's solder fusible at a low temperature is prepared by melting together:
I. Tin, 2 parts; lead, 1 part; bismuth, 1 part; melting point, 266º F.
II. Tin, 3 parts; lead, 4 parts; bismuth, 2 parts; melting point, 297º F.
III. Tin, 2 parts; lead, 2 parts; bismuth, 1 part; melting point, 320º F.
STEEL SOLDERING.
Dissolve scraps of cast steel in as small a quantity as possible of nitric acid, add finely pulverized borax and stir vigorously until a fluid paste is formed, then dilute by means of sal ammoniac and put in a bottle. When soldering is to be done, apply a thin layer of the solution to the two parts to be soldered, and when these have been carried to ordinary redness, and the mass is consequently plastic, beat lightly on the anvil with a flat hammer. This recipe is useful for cases when the steel is not to be soldered at an elevation of temperature to the bright red.
To Solder a Piece of Hardened Steel. To hard solder a piece of hardened steel such as index (regulator), stop spring (in the part which is not elastic), click, etc., take a very flat charcoal if the piece is difficult to attach; hard-solder and as soon as the soldering has been done, plunge the piece into oil. All that remains to be done is to blue it again and to polish.
Soldering Powder for Steel. Melt in an earthen pot 3 parts of borax, 2 of colophony, 1 of potassium carbonate, as much powdered hard soap, to which must be added 3 parts of finely powdered glass and 2 parts of steel filings. The melted mass is run out upon a cold plate of sheet iron, and when it is completely chilled it is broken into small bits or finely powdered. To solder, it is necessary to sprinkle the powder on the surfaces to be joined several minutes before bringing them together.
Soldering Solution for Steel. A soldering solution for steel that will not rust or blacken the work is made of 6 ounces alcohol, 2 ounces glycerine, and 1 ounce oxide of zinc.
PLATINUM SOLDERS.
There are many platinum solders in existence, but the main principle to be borne in mind in jewelry work is that the soldering seam should be as little perceptible as possible; the solder, therefore, should have the same color as the alloy.
I. A platinum solder which meets these requirements very satisfactorily is composed of 9 parts gold and 1 part palladium; or, 8 parts gold and 2 parts palladium.
II. The following is a readily fusible platinum solder: Fine silver, 1.555 parts, and pure platinum, 0.583 parts. This melts easily in the ordinary draught furnace, as well as before the soldering pipe on a piece of charcoal. Of similar action is a solder of the following composition, which is very useful for places not exposed to the view:
III. Fine gold, 1.555 parts; fine silver, 0.65 parts; and pure copper, 0.324 parts.
SOLDER FOR IRON:
See also under Silver Solders. Copper, 67 parts; zinc, 33 parts; or, copper, 60 parts; zinc, 40 parts.
TIN SOLDERS:
See also Soft Solders.
Gold jewelry which has been rendered unsightly by tin solder may be freed from tin entirely by dipping the article for a few minutes into the following solution and then brushing off the tin: Pulverize 2 parts of green vitriol and 1 part of saltpeter and boil in a cast-iron pot with 10 parts of water until the irger part of the latter has evaporated. The crystals forming upon cooling are dissolved in hydrochloric acid (8 parts of hydrochloric acid to 1 part of crystals). If the articles in question have to be left in the liquid for some time, it is well to dilute it with 3 or 4 parts of water. The tin solder is dissolved by this solution without attacking or damaging the article in the least.
VARIOUS RECIPES FOR SOLDERING:
To Conceal Soldering. Visible soldering may be obviated by the following methods: For copper goods a concentrated solution of blue vitriol is prepared and applied to the places by means of an iron rod or iron wire. The thickness of
[666]
SOLDERS
the layer may be increased by a repetition of the process. In order to give the places thus coppered the appearance of the others, use a saturated solution of zinc vitriol, 1 part, and blue vitriol, 2 parts, and finish rubbing with a piece of zinc. By sprinkling on gold powder and subsequently polishing, the color is rendered deeper. In the case of gold articles the places are* first coppered over, then covered with a thin layer of fish glue, after which bronze filings are thrown on. When the glue is dry rub off quickly to produce a fine polish. The places can, of course, also be electro-gilt, whereby a greater uniformity of the shade is obtained. In silver objects, the soldering seams, etc., are likewise coppered in the above-described manner; next they are rubbed with a brush dipped into silver powder and freshly polished.
Solder for Articles which will not Bear a High Temperature. Take powdered copper, the precipitate of a solution of the sulphate by means of zinc, and mix it with concentrated sulphuric acid. According to the degree of hardness required, take from 20 to 30 or 36 parts of copper. Add, while constantly snaking, 70 parts of quicksilver, and when the amalgam is complete, wash with warm water to remove the acid; then allow it to cool. In 10 or 12 hours the composition will be hard enough to scratch tin. For use, warm it until it reaches the consistency of wax, and spread it where needed. When cold it will adhere with great tenacity.
Soldering a Ring Containing a Jewel.
I. Fill a small crucible with wet sand and bury the part with the jewel in the sand. Now solder with soft gold solder, holding the crucible in the hand. The stone will remain uninjured.
II. Take tissue paper, tear it into strips about 3 inches in width, and make them into ropes; wet them thoroughly and wrap the stone in them, passing around the stone and through the ring until the center of the latter is slightly more than half filled with paper, closely wound around. Now fix on charcoal, permitting the stone to protrude over the edge of the charcoal, and solder rapidly. The paper will not only protect the stone, but also prevent oxidation of the portion of the ring which is covered.
Soldering without Heat. For soldering objects without heating, take a large copper wire filed to a point; dip into soldering water and rub the parts to be soldered. Then heat the copper wire and apply the solder, which melts on contact. It may then be applied to the desired spot without heating the object.
COLD SOLDERING:
See also Adhesives and Cements.
For soldering articles which cannot stand a high temperature, the following process may be employed:
I. Take powdered copper precipitated from a solution of sulphate by means of zinc and mix it in a cast-iron or porcelain mortar with concentrated sulphuric acid. The number of parts of copper varies according to the degree of hardness which it is wished to obtain. Next add, stirring constantly, 70 parts of mercury, and when the amalgam is finished, allow to cool. At the end of 10 to 12 hours the composition is sufficiently hard. For use, heat until it acquires the consistency of wax. Apply to the surface. When cool it will adhere with great tenacity.
II. Crush and mix 6 parts of sulphur, 6 parts of white lead, and 1 part of borax. Make a rather thick cement of this powder by triturating it with sulphuric acid. The paste is spread on the surfaces to be welded, and the articles pressed firmly together. In 6 or 7 days the soldering is so strong that the two pieces cannot be separated, even by striking them with a hammer.
Cast-iron Soldering. A new process consists in decarbonizing the surfaces of the cast iron to be soldered, the molten hard solder being at the same time brought into contact with the red hot metallic surfaces. The admission of air, however, should be carefully guarded against. First pickle the surfaces of the pieces to be soldered, as usual, with acid and fasten the two pieces together. The place to be soldered is now covered with a metallic oxygen compound and any one of the customary fluxes and heated until red hot. The preparation best suited for this purpose is a paste made by intimately mingling together cuprous oxide and borax. The latter melts in soldering and protects the pickled surfaces as well as the cuprous oxide from oxidation through the action of the air. During the heating the cuprous oxide imparts its oxygen to the carbon contained in the cast iron and burns it. Metallic copper separates in fine subdivision. Now apply hard solder to the place to be united, which in melting forms an alloy with the eliminated copper, the alloy combining with the decarburized surfaces of the cast iron.
[667]
SOLDERS - SPIRIT
Soldering Block. This name is given to a very useful support for hard soldering and can be readily made. The ingredients are: Charcoal, asbestos, and plaster of Paris. These are powdered in equal parts, made into a thick paste with water, and poured into a suitable mold. Thus a sort of thick plate is obtained. When this mass has dried it is removed from the mold and a very thin cork plate is affixed on one surface by means of thin glue. The mission of this plate is to receive the points of the wire clamps with which the articles to be soldered are attached to the soldering block, the asbestos not affording sufficient hold for them.
SOLDERS FOR JEWELERS:
See Jewelers' Formulas.
SOLDER FROM GOLD, TO REMOVE:
See Gold.
SOLDERING PASTE.
The semi liquid mass termed soldering paste is produced by mixing zinc chloride solution or that of ammonia zinc chloride with starch paste. For preparing this composition, ordinary potato starch is made with water into a milky liquid, the latter is heated to a boil with constant stirring, and enough of this mass, which becomes gelatinous after cooling, is added to the above-mentioned solutions as to cause a liquid resembling thin syrup to result. The use of all zinc preparations for soldering presents the drawback that vapors of a strongly acid odor are generated by the heat of the soldering iron, but this evil is offset by the extraordinary convenience afforded when working with these preparations. It is not necessary to subject the places to be soldered to any special cleaning or preparation. All that is required is to coat them with the soldering medium, to apply the solder to the seam, etc., and to wipe the places with a sponge or moistened rag after the solder has cooled. Since the solder adheres readily with the use of these substances, a skillful workman can soon reach such perfection that he has no, or very little, subsequent polishing to do on the soldering seams.
Soft Soldering Paste. Small articles of any metals that would be very delicate to solder with a stick of solder, especially where parts fit into another and only require a little solder to hold them together, can best be joined with a soldering paste. This paste contains the solder and flux combined, and is easily applied to seams, or a little applied before the parts are put together. The soldering flame will cause the tin in the paste to amalgamate quickly. The paste is made out of starch paste mixed with a solution of chloride of tin to the consistency of syrup.
SOLUTIONS, PERCENTAGE:
See Tables.
SOOTHING SYRUP:
See Pain Killers.
SOUP HERB EXTRACT:
See Condiments.
SOZODONT:
See Dentifrices.
SPARKS FROM THE FINGER TIPS:
See Pyrotechnics.
SPATTER WORK:
See Lettering.
SPAVIN CURES:
See Veterinary Formulas.
SPECULUM METAL:
See Alloys.
SPICES, ADULTERATED:
See Foods.
SPICES FOR FLAVORING:
See Condiments.
Spirit
INDUSTRIAL AND POTABLE ALCOHOL: SOURCES AND MANUFACTURE.
Abstract of a Farmers' Bulletin prepared for the United States Department of Agriculture by Dr. Harvey W. Wiley.
The term "industrial alcohol," or spirit, is used for brevity, and also because it differentiates sharply between alcohol used for beverages or for medicine and alcohol used for technical purposes in the arts.
Alcohol Defined. The term "alcohol" as here used and as generally used means that particular product which is obtained by the fermentation of a sugar, or a starch converted into sugar, and which, from a chemical point of view, is a compound of the hypothetical substance "ethyl" with water, or with that part of water remaining after the separation of one of the atoms of hydrogen. This is a rather technical expression, but it is very difficult, without using technical language, to give a definition of alcohol from the chemical point of view. There are three elementary substances represented in alcohol: Carbon, the chemical symbol of which is C; hydrogen, symbol
[668]
SPIRIT
H; and oxygen, symbol O. These atoms are put together to form common alcohol, or, as it is called, ethyl alcohol, in which preparation 2 atoms of carbon and 5 atoms of hydrogen form the hypothetical substance "ethyl," and 1 atom of oxygen and 1 atom of hydrogen form the hydroxyl derived from water. The chemical symbol of alcohol therefore is C2H6OH. Absolutely pure ethyl alcohol is made only with great difficulty, and the purest commercial forms still have associated with them traces of other volatile products formed at the time of the distillation, chief among which is that group of alcohols to which the name "fused oil" is applied. So far as industrial purposes are concerned, however, ethyl alcohol is the only component of any consequence, just as in regard to the character of beverages the ethyl alcohol is the component of least consequence.
Sources of Potable Alcohol. The raw materials from which alcohol is made consist of those crops which contain sugar, starch, gum, and cellulose (woody fiber) capable of being easily converted into a fermentable sugar. Alcohol as such is not used as a beverage. The alcohol occurring in distilled beverages is principally derived from Indian corn, rye, barley, and molasses. Alcohol is also produced for drinking purposes from fermented fruit juices such as the juice of grapes, apples, peaches, etc. In the production of alcoholic beverages a careful selection of the materials is required in order that the desired character of drink may be secured. For instance, in the production of rum, the molasses derived from the manufacture of sugar from sugar cane is the principal raw material. In the fermentation of molasses a particular product is formed which by distillation gives the alcohol compound possessing the aroma and flavor of rum. In the making of brandy, only sound wine can be used as the raw material, and this sound wine, when subjected to distillation, gives a product containing the same kind of alcohol as that found in rum, but associated with the products of fermentation which give to the distillate a character entirely distinct and separate from that of rum. Again, when barley malt or a mixture of barley malt and rye is properly mashed, fermented, and subjected to distillation, a product is obtained which, when properly concentrated and aged, becomes potable malt or rye whisky. In a similar manner, if Indian corn and barley malt are properly mashed, with a small portion of rye, the mash fermented and subjected to distillation, and the distillate properly prepared and aged, the product is known as Bourbon whisky. Thus, every kind of alcoholic beverage gets its real character, taste, and aroma, not from the alcohol which it contains but from the products of fermentation which are obtained at the same time the alcohol is made and which are carried over with the alcohol at the time of distillation.
Agricultural Sources of Industrial Alcohol. The chief alcohol-yielding material produced in farm crops is starch, the second important material is sugar, and the third and least important raw material is cellulose, or woody fiber. The quantity of alcohol produced from cellulose is so small as to be of no importance at the present time, and therefore this source of alcohol will only be discussed under the headings "Utilization of Waste Material or By-Products" and "Wood Pulp and Sawdust."
Starch Producing Plants. Starch is a compound which, from the chemical point of view, belongs to the class known as carbohydrates, that is, compounds in which the element carbon is associated by a chemical union with water. Starch is therefore a compound made of carbon, hydrogen, and oxygen, existing in the proportion of 2 atoms of hydrogen to 1 atom of oxygen. Each molecule of starch contains at least 6 atoms of carbon, 10 atoms of hydrogen, and 5 atoms of oxygen. The simplest expression for starch is therefore C6H10O5. Inasmuch as this is the simplest expression for what the chemist knows as a molecule of starch, and it is very probable that very many, perhaps a hundred or more, of these molecules exist together, the proper expression for starch from a chemical point of view would be (C6H10O5)X.
The principal starch-producing plants are the cereals, the potato, and cassava. With the potato may be classed, though not botanically related thereto, the sweet potato and the yam. Among cereals rice has the largest percentage of starch and oats the smallest. The potato, as grown for the table, has an average content of about 15 per cent of starch. When a potato is grown specifically for the production of alcohol it contains a larger quantity, or nearly 20 per cent. Cassava contains a larger percentage of starch than the potato, varying from 20 to 30 per cent.
Sugar Producing Plants. Sugar cans,
[669]
SPIRIT
etc. While sugar is present in some degree in all vegetable growths, there are some plants which produce it in larger quantities than are required for immediate needs, and this sugar is stored in some part of the plant. Two plants are preeminently known for their richness in sugar, namely, the sugar cane and the sugar beet. In Louisiana the sugar canes contain from 9 to 14 per cent of sugar, and tropical canes contain a still larger amount.
The juices of the sugar beet contain from 12 to 18 per cent of sugar. There are other plants which produce large quantities of sugar, but which are less available for sugar-making purposes than those just mentioned. Among these, the sorghum must be first mentioned, containing in the stalk at the time the seed is just mature and the starch hardened from 9 to 15 per cent of sugar. Sorghum seed will also yield as much alcohol as equal weights of Indian corn. The juices of the stalks of Indian corn contain at the time the grain is hardening and for some time thereafter large quantities of sugar, varying from 8 to 15 per cent.
In the case of the sorghum and the Indian-corn stalk a large part of the sugar present is not cane sugar or sucrose as it is commonly known, but the invert sugar derived therefrom. For the purposes of making alcohol the invert sugar is even more suitable than cane sugar. Many other plants contain notable quantities of sugar, but, with the exception of fruits, discussed under the following caption, not in sufficient quantities to be able to compete with those just mentioned for making either sugar or alcohol.
Cane sugar is not directly susceptible to fermentation. Chemically considered, it has the formula expressed by the symbols: C12H22O11. When cane sugar having the above composition becomes inverted, it is due to a process known as hydrolysis, which consists in the molecule of cane sugar taking up 1 molecule of water and splitting off into 2 molecules of sugar having the same formula but different physical and chemical properties. Thus the process may be represented as follows: C12H22O11 (cane sugar) + H2O (water) = C6H12O6 (dextrose) + C6H12O6 (levulose). These two sugars (dextrose and levulose) taken together are known as invert sugar and are directly susceptible to fermentation. All cane sugar assumes the form of invert sugar before it becomes fermented.
Fruits. Nearly all fruit juices are rich in sugar, varying in content from 5 to 30 per cent. The sugar in fruits is composed of both cane sugar and its invert products (dextrose and levulose), in some fruits principally the latter. Of the common fruits the grape yields the largest percentage of sugar. The normal grape used for wine making contains from 16 to 30 per cent of sugar, the usual amount being about 20 per cent. Fruit juices are not usually employed in any country for making industrial alcohol, because of their very much greater value for the production of beverages.
Composition and Yield of Alcohol Producing Crops. The weight of alcohol that may be produced from a given crop is estimated at a little less than one-half of the amount of fermentable substance present, it being understood that the fermentable substance is expressed in terms of sugar. Pasteur was the first to point out the fact that when sugar was fermented it yielded theoretically a little over one-half of its weight of alcohol. It must be remembered, however, that in the production of alcohol a process of hydrolysis is taking place which adds a certain quantity of alcohol to the products which are formed. For this reason 100 parts of sugar yield more than 100 parts of fermentable products. The distribution of the weights produced, as theoretically calculated by Pasteur, is as follows:
One hundred parts of sugar yield the following quantities of the products of fermentation:
Alcohol 51.10 parts
Carbonic acid 49.20 parts
Glycerine 3.40 parts
Organic acids, chiefly succinic 65 parts
Ethers, aldehydes, furfural,
fat, etc. 1.30 parts
------------
Total weight fermentation
products produced 105.65 parts
Artichokes. The artichoke has been highly recommended for the manufacture of alcohol. The fermentable material in the artichoke is neither starch nor sugar, but consists of a mixture of a number of carbohydrates of which inulin and levulin are the principal constituents. When these carbohydrate materials are hydrolized into sugars they produce levulose instead of dextrose. The levulose is equally as valuable as dextrose for the production of alcohol. Artichokes may be harvested either in the autumn or in the spring. As they keep well during the winter, and in a few places
[670]
SPIRIT
may be kept in hot weather, they form a raw material which can be stored for a long period and still be valuable for fermentation purposes.
Under the term "inulin" are included all the fermentable carbohydrates. The above data show, in round numbers, 17 per cent of fermentable matter. Theoretically, therefore, 100 pounds of artichokes would yield approximately 8 1/2 pounds of industrial alcohol, or about 1 1/4 gallons.
Bananas. The banana is a crop which grows in luxurious abundance in tropical countries, especially Guatemala and Nicaragua. The fruit contains large quantities of starch and sugar suitable for alcohol making. From 20 to 25 per cent of the weight of the banana consists of fermentable material. It is evident that in the countries where the banana grows in such luxuriance it would be a cheap source of industrial alcohol.
Barley and the Manufacture of Malt. A very important cereal in connection with the manufacture of alcohol is barley which is quite universally employed for making malt, the malt in its turn being used for the conversion of the starch of other cereals into sugar in their preparation for fermentation.
Malt is made by the sprouting of barley at a low temperature (from 50 to 60º F.) until the small roots are formed and the germ has grown to the length of 1/2 an inch or more. The best malts are made at a low temperature requiring from 10 to 14 days for the growth of the barley. The barley is moistened and spread upon a floor, usually of cement, to the depth of 1 foot or 18 inches. As the barley becomes warm by the process of germination, it is turned from time to time and the room is kept well ventilated and cool. It is better at this point in the manufacture of malt to keep the temperature below 60º F. After the sprouting has been continued as above noted for the proper length of time, the barley is transferred to a drier, where it is subjected to a low temperature at first and finally to a temperature not to exceed 140º or 158º F., until all the water is driven off, except 2 or 3 per cent. Great care must be exercised in drying the barley not to raise the temperature too high, lest the diastase which is formed be deprived of its active qualities. The malt has a sweetish taste, the principal portion of the starch having been converted into sugar, which is known chemically as "maltose." This sugar is, of course, utilized in the fermentation for the production of alcohol. Malt is chiefly valuable, however, not because of the amount of alcohol that may be produced therefrom, but from the fact that in quantities of about 10 per cent it is capable of converting the starch of the whole of the unmalted grains, whatever their origin may be, into maltose, thus preparing the starch for fermentation, barley is not itself used in this country as a source of industrial alcohol, but it is employed for producing the highest grades of whisky, made of pure barley malt, which, after fermentation, is distilled in a pot still, concentrated in another pot still to the proper strength, placed in wood, and stored for a number of years. Barley malt is too expensive a source of alcohol to justify its use for industrial purposes. It is, however, one of the cheapest and best methods of converting the starch of other cereals into sugar preparatory to fermentation. Barley has, in round numbers, about 68 per cent of fermentable matter. The weight of a bushel of barley (48 pounds) multiplied by 0.68 gives 32 pounds of fermentable matter in a bushel of barley.
Cassava. Cassava is grown over a large area of the South Atlantic and Gulf States of this country. Of all the substances which have been mentioned, except the cereals, cassava contains the largest amount of alcoholic or fermentable substances. The root, deprived of its outer envelope, contains a little over 30 per cent of starch, while the undetermined matter in the analyses is principally sugar. If this be added to the starch, it is seen that approximately 35 per cent of the fresh root is fermentable. This of course represents a very high grade of cassava, the ordinary roots containing very much less fermentable matter. If, however, it is assumed that the fermentable matter of cassava root will average 25 per cent, this amount is much greater than the average of the potato, or even of the sweet potato and the yam. Twenty-five per cent is undoubtedly a low average content of fermentable matter. In the dry root there is found nearly 72 per cent of starch and 17 per cent of extract, principally sugar. Assuming that 15 per cent of this is fermentable, and adding this to the 72 per cent, it is seen that 87 per cent of the dry matter of the cassava is fermentable. This appears to be a very high figure, but it doubtless represents almost exactly the conditions which exist. It would be perfectly safe to say, discounting any exceptional qualities of the samples examined, that 80 per cent of the dry matter of the cassava root is
[671]
SPIRIT
capable of being converted into alcohol. It thus becomes in a dry state a source of alcohol almost as valuable, pound for pound, as rice.
Careful examinations, however, of actual conditions show that if 5 tons per acre of roots are obtained it is an average yield. In very many cases, where no fertilizer is used and where the roots are grown in the ordinary manner, the yield is far less than this, while with improved methods of agriculture it is greater. The bark of the root, has very little fermentable matter in it. If the whole root be considered, the percentage of starch is less than it would be for the peeled root. If cassava yields 4 tons, or 8,000 pounds, per acre and contains 25 per cent of fermentable matter, the total weight of fermentable matter is 2,000 pounds, yielding approximately 1,000 pounds of 95 per cent alcohol, or 143 gallons of 95 per cent alcohol per acre.
Corn (Indian Corn or Maize). The crop which at the present time is the source of almost all of the alcohol made in the United States is Indian corn.
The fermentable matter in Indian corn that is, the part which is capable of being converted into alcohol amounts to nearly 70 per cent of the total weight, since the unfermentable cellulose and pentosans included in carbohydrates do not exceed 2 per cent. Inasmuch as a bushel of Indian corn weighs 56 pounds, the total weight of fermentable matter therein, in round numbers, is 39 pounds. The weight of the alcohol which is produced under the best conditions is little less than one-half of the fermentable matter. Therefore the total weight of alcohol which would be yielded by a bushel of average Indian corn would be, in round numbers, about 19 pounds. The weight of a gallon of 95 per cent alcohol is nearly 7 pounds. Hence 1 bushel of corn would produce 2.7 gallons.
If the average price of Indian corn be placed, in round numbers, at 40 cents a bushel, the cost of the raw material that is, of the Indian corn for manufacturing 95 per cent industrial alcohol is about 15 cents a gallon. To this must be added the cost of manufacture, storage, etc., which is perhaps as much more, making the estimated actual cost of industrial alcohol of 95 per cent strength made from Indian corn about 30 cents per gallon. If to this be added the profits of the manufacturer and dealer, it appears that under the conditions cited, industrial alcohol, untaxed, should be sold for about 40 cents per gallon.
Potatoes. The weight of a bushel of potatoes is 60 pounds. As the average amount of fermentable matter in potatoes grown in the United States is 20 per cent, the total weight of fermentable matter in a bushel of potatoes is 12 pounds, which would yield approximately 6 pounds or 3.6 quarts of alcohol.
The quantity of starch in American grown potatoes varies from 15 to 20 per cent. Probably 18 per cent might be stated as the general average of the best grades of potatoes.
Under the microscope the granules of potato starch have a distinctive appearance. They appear as egg-shaped bodies on which, especially the larger ones, various ring-like lines are seen. With a modified light under certain conditions of observation a black cross is developed upon the granule. It is not difficult for an expert microscopist to distinguish potato from other forms of starch by this appearance.
The potato contains very little material which is capable of fermentation aside from starch and sugars.
Although the potato is not sweet to the taste in a fresh state, it contains notable quantities of sugar. This sugar is lost whenever the potato is used for starch-making purposes, but is utilized when it is used for the manufacture of industrial alcohol. The percentage of sugar of all kinds in the potato rarely goes above 1 per cent. The average quantity is probably not far from 0.35 per cent, including sugar, reducing sugar, and dextrin, all of which are soluble in water. In the treatment of potatoes for starch making, therefore, it may be estimated that 0.35 per cent of fermentable matter is lost in the wash water.
Average Composition. The average composition of potatoes is:
Water 75.00 per cent
Starch 19.87 per cent
Sugars and dextrin .77 per cent
Fat .08 per cent
Cellulose .33 per cent
Ash 1.00 per cent
According to Maercker, the sugar content, including all forms of sugar, varies greatly. Perfectly ripe potatoes contain generally no sugar or only a fractional per cent. When potatoes are stored under unfavorable conditions, large quantities of sugar may be developed, amounting to as high as 5 per cent altogether. In general, it may be stated that the content of sugar of all kinds will vary from 0.4 per cent to 3.4 per cent, according to conditions.
[672]
SPIRIT
The liberal application of nitrogenous fertilizers increases the yield per acre of tubers and of starch to a very marked extent, although the average percentage of starch present is increased very little.
Of all the common root crops, the potatoes, including the yam and the sweet potato, are the most valuable for the production of alcohol, meaning by this term that they contain more fermentable matter per 100 pounds than other root crops.
While sugar beets, carrots, and parsnips contain relatively large amounts of fermentable matter, these roots could not compete with potatoes even if they could all be produced at the same price per 100 pounds.
A general review of all the data indicates that under the most favorable circumstances and with potatoes which have been grown especially for the purpose an average content of fermentable matter of about 20 per cent may be reasonably expected. It is thus seen that approximately 10 pounds of industrial alcohol can be made from 100 pounds of potatoes. If 60 pounds be taken as the average weight of a bushel of potatoes, there are found therein 12 pounds of fermentable matter, from which 6 pounds of industrial alcohol can be produced, or 6/7 of a gallon. It has also been shown that the amount of Indian corn necessary for the production of a gallon of industrial alcohol costs not less than 15 cents. From this it is evident that the potatoes for alcohol making will have to be produced at a cost not to exceed 15 cents per bushel, before they can compete with Indian corn for the manufacture of industrial alcohol.
Rice. Rice is not used to any great extent in this country for making alcohol, but it is extensively used for this purpose in Japan and some other countries, and has the largest percentage of fermentable matter of all the cereals. The percentage of fermentable matter in rice is nearly 78 per cent. A bushel of rice weighs, unhulled, 45 pounds, hulled, 56 pounds, and it therefore has about 34 and 43 pounds, respectively, of fermentable matter for the unhulled and the hulled rice. It is not probable that rice will ever be used to any extent in this country as a source of industrial alcohol, although it is used to a large extent in the manufacture of beverages, as for instance in beers, which are often made partly of rice.
Rye. Large quantities of alcohol, chiefly in the form of alcoholic beverages, are manufactured from rye. It is, in connection with Indian corn, the principal source of the whiskies made in the United States. Rye, however, is not used to any extent in this or other countries for making industrial alcohol.
Rye contains almost as much fermentable matter as Indian corn. A bushel of rye weighs 56 pounds. Wheat and other cereals, not mentioned above, are not used in this country to any appreciable extent in the manufacture of alcohol.
Spelt. This grain, which is botanically a variety of wheat, more closely resembles barley. Under favorable conditions as much as 73 bushels per acre have been reported, and analyses show 70 per cent of fermentable carbohydrates. The weight per bushel is about the same as that of oats. It would appear that this crop might be worthy of consideration as a profitable source of industrial alcohol.
Sugar Beets. The sugar beet is often used directly as a source of alcohol. Working on a practical scale in France, it has been found that from 10,430 tons of beets there were produced 183,624 gallons of crude alcohol of 100 per cent strength. The beets contain 11.33 per cent of sugar. From 220 pounds of sugar 15.64 gallons of alcohol were produced. The weight of pure alcohol obtained is a little less than one-half the weight of the dry fermentable matter calculated as sugar subjected to fermentation. About 18 gallons of alcohol are produced for each ton of sugar beets employed.
Sweet Potatoes. Experiments show that as much as 11,000 pounds of sweet potatoes can be grown per acre. The average yield of sweet potatoes, of course, is very much less. On plots to which no fertilizer is added the yield is about 8,000 pounds of sweet potatoes per acre, yielding in round numbers 1,900 pounds of starch. The quantity of sugar in the 8,000 pounds is about 350 pounds, which added to the starch, makes 2,250 pounds of fermentable matter per acre. This will yield 1,125 pounds of industrial alcohol of 95 per cent strength, or approximately 160 gallons per acre. The percentage of starch is markedly greater than in the white or Irish potato. In all cases over 20 per cent of starch was obtained in the South Carolina sweet potatoes, and in one instance over 24 per cent. As much as 2,600 pounds of starch were produced per acre.
In addition to starch, the sweet potato contains notable quantities of sugar, sometimes as high as 6 per cent being present, so that the total fermentable matter in the sweet potato may be reck-
[673]
SPIRIT
oned at the minimum at 25 per cent. A bushel of sweet potatoes weighs 55 pounds, and one-quarter of this is fermentable matter, or nearly 14 pounds. This would yield, approximately, 7 pounds, or a little over 1 gallon of 95 per cent alcohol. It may be fairly stated, therefore, in a general way, that a bushel of sweet potatoes will yield 1 gallon of industrial alcohol.
Experiments have shown that the quantity of starch diminishes and the quantity of sugar increases on storing. Further, it may be stated that in the varieties of sweet potatoes which are most esteemed for table use there is less starch and perhaps more sugar than stated above. The total quantity of fermentable matter, however, does not greatly change, although there is probably a slight loss.
Utilization of Waste Material or Byproducts.
Molasses. The utilization of the waste materials from the sugar factories and sugar refineries for the purpose of making alcohol is a well-established industry. The use of these sources of supply depends, of course, upon the cost of the molasses. When the sugar has been exhausted as fully as possible from the molasses the latter consists of a saccharine product, containing a considerable quantity of unfermentable carbohydrate matter, large quantities of mineral salts, and water. In molasses of this kind there is probably not more than 50 pounds of fermentable matter to 100 pounds of the product. Assuming that a gallon of such molasses weighs 11 pounds, it is seen that it contains 5 1/2 pounds of fermentable matter, yielding 2 1/4 pounds of industrial alcohol of 95 per cent strength. It requires about 3 gallons of such molasses to make 1 gallon of industrial alcohol.
When the price of molasses delivered to the refineries falls as low as 5 or 6 cents a gallon it may be considered a profitable source of alcohol.
Wood Pulp and Sawdust. Many attempts have been made to produce alcohol for industrial purposes from sawdust, wood pulp, or waste wood material. The principle of the process rests upon the fact that the woody substance is composed of cellulose and kindred matters which, under the action of dilute acid (preferably sulphuric or sulphurous) and heat, with or without pressure, undergo hydrolysis and are changed into sugars. A large part of the sugar which is formed is nonfermentable, consisting of a substance known as xylose. Another part of the sugar produced is dextrose, made from the true cellulose which the wood contains.
The yield of alcohol in many of the experiments which have been made has not been very satisfactory. It is claimed, however, by some authors that paying quantities of alcohol are secured. In Simmonsen's process for the manufacture of alcohol 1/2 per cent sulphuric acid is employed and from 4 to 5 parts of the liquid heated with 1 part of the finely comminuted wood for a quarter of an hour under a pressure of 9 atmospheres. It is claimed by Simmonsen that he obtained a yield of 6 quarts of alcohol from 110 pounds of air-dried shavings. Another process which has been tried in this and other countries for converting comminuted wood into alcohol is known as Classen's. The comminuted wood is heated for 15 minutes in a closed apparatus at a temperature of from 248 to 293º F. in the presence of sulphurous acid (fumes of burning sulphur) instead of sulphuric acid. It is claimed by the inventor that he has made as much as 12 quarts of alcohol from 110 pounds of the air-dried shavings. There is reason to doubt the possibility of securing such high yields in actual practice as are claimed in the above processes. That alcohol can be made from sawdust and wood shavings is undoubtedly true, but whether or not it can be made profitably must be determined by actual manufacturing operations.
Waste Products of Canneries, etc. The principal waste materials which may be considered in this connection are the refuse of wine making, fruit evaporating, and canning industries, especially the waste of factories devoted to the canning of tomatoes and Indian corn. In addition to this, the waste fruit products themselves, which are not utilized at all, as, for instance, the imperfect and rotten apples, tomatoes, grapes, etc., may be favorably considered. The quantity of waste products varies greatly in different materials.
The quantities of waste material in grapes and apples, as shown by Lazenby, are as follows: About 25 per cent of the total weight in grapes, with the exception of the wild grape, where it is about 60 per cent; with apples the average percentage of waste was found to be 23.8 per cent from 25 varieties. This included the waste in the core, skin, and the defective apples caused by insects, fungi/bruises, etc. In general it may be said that in the preparation of fruits for
[674]
SPIRIT
preserving purposes about 25 per cent of their weight is waste, and this, it is evident, could be utilized for the manufacture of alcohol. If apples be taken as a type of fruits, we may assume that the waste portions contain 10 per cent of fermentable matters, which, however, is perhaps rather a high estimate. Five per cent of this might be recovered as industrial alcohol. Thus, each 100 pounds of fruit waste in the most favorable circumstances might be expected to produce 5 pounds of industrial alcohol. The quantity of waste which could be utilized for this purpose would hardly render it profitable to engage in the manufacture. A smaller percentage could be expected from the waste of the tomato, where the quantity of sugar is not so great. In the waste of the sweetcorn factory the amount of fermentable matter would depend largely on the care with which the grain was removed. There is usually a considerable quantity of starchy material left on the cobs, and this, with the natural sugars which the grown cobs contain, might yield quite large Quantities of fermentable matter. It would not be profitable to erect distilleries simply for the utilization of waste of this kind, but if these wastes could be utilized in distilleries already established it might be profitable to devote them to this purpose.
Manufacture of Alcohol. The three principal steps in the manufacture of alcohol are (1) the preparation of the mash or wort, (2) the fermentation of the mash or wort drawn off from the mash tun, and (3) the distillation of the dilute alcohol formed in the beer or wash from the fermentation tanks. The preparation of the mash includes (1) the treatment of the material used with hot water to form a paste of the starch or the sugar, and (2) the action of the malt or ferment on the paste to convert the starch into fermentable sugar.
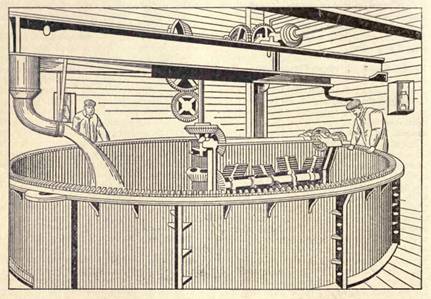
FIG. 1. MASH TUN IN AN IRISH DISTILLERY.
Mashing. Figs. 1 and 2 show two views of the mashing tun or tank, the first figure giving the general appearance, and the second a view of the interior of the tun, showing the machinery by which the stirring is effected and the series of pipes for cooling the finished product down to the proper temperature for the application of the malt.
The object of the mash tun is to reduce the starch in the ground grain to a pasty, gummy mass, in order that the ferment of the malt rnay act upon it vigorously and convert it into sugar. If the mashing be done before the addition
SPIRIT
[675]
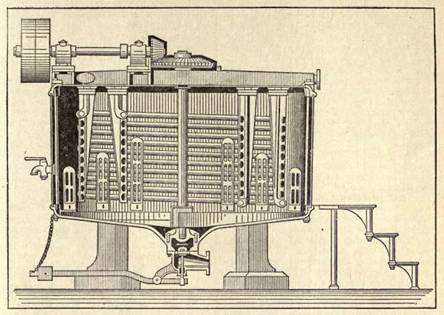
FIG. 2. MASHING AND COOLING APPARATUS, CROSS SECTION.
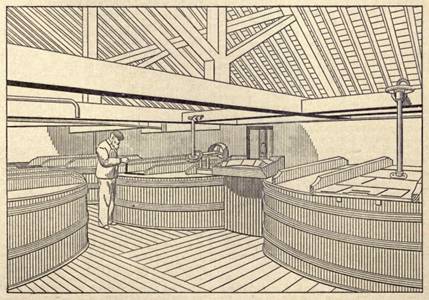
FIG. 3. FERMENTATION TANKS IN AN IRISH DISTILLERY.