 The
Science Notebook
The
Science NotebookHenley's Book of Formulas, Recipes and Processes
Home Terms of Use Safety Contact Us Experiment Pages Downloads Supplies Useful Links!
Henley's Twentieth Century Book of Formulas, Recipes and Processes - Pages 726-750
[726]
VARNISHES
and 1,700 parts, by weight, of spirit; digest in a warm place 24 hours, and filter. Next dissolve 80 parts, by weight, of dragon's blood; 80 parts, by weight, of sandarac; 80 parts, by weight, of elemi gum; 50 parts, by weight, of gamboge; 70 parts, by weight, of seedlac. Mix these substances with 250 parts, by weight, of crushed glass, place them in a flask, and pour over this mixture the alcohol colored as above described. Assist the solution by means of a sand or water bath, and filter at the close of the operation. This is a fine varnish for brass scientific instruments.
Bronze Varnishes.
I. The following process yields a top varnish for bronze goods and other metallic ware in the most varying shades, the varnish excelling, besides, in high gloss and durability. Fill in a bottle, pale shellac, best quality, 40 parts, by weight; powdered Florentine lake, 12 parts, by weight; gamboge, 30 parts, by weight; dragon's blood, also powdered, 6 parts, by weight; and add 400 parts, by weight, of spirit of wine. This mixture is allowed to dissolve, the best way being to heat the bottle on the water bath until the boiling point of water is almost reached, shaking from time to time until all is dissolved. Upon cooling, decant the liquid, which constitutes a varnish of dark-red color, from any sediment that may be present. In a second bottle dissolve in the same manner 24 parts, by weight, of gamboge in 400 parts, by weight, of spirit of wine, from which will result a varnish of golden-yellow tint. According to the hue desired, mix the red varnish with the yellow variety, producing in this way any shade from the deepest red to the color of gold. If required, dilute with spirit of wine. The application of the varnish should be conducted as usual, that is, the article should be slightly warm, it being necessary to adhere strictly to a certain temperature, which can be easily determined by trials and maintained by experience. In order to give this varnish a pale-yellow to greenish-yellow tone, mix 10 drops of picric acid with about 3 parts, by weight, of spirit of wine, and add to a small quantity of the varnish some of this mixture until the desired shade has been reached. Picric acid is poisonous, and the keeping of varnish mixed with this acid in a closed bottle is not advisable, because there is danger of an explosion. Therefore, it is best to prepare only so much varnish at one time as is necessary for the immediate purpose.
Brown Varnish. An excellent and quickly drying brown varnish for metals is made by dissolving 20 ounces of gum kino and 5 ounces of gum benjamin in 60 ounces of the best cold alcohol; 20 ounces of common shellac and 2 ounces of thick turpentine in 36 ounces of alcohol also give a very good varnish. If the brown is to have a reddish tint, dissolve 50 ounces of ruby shellac, 5 ounces balsam of copaiba, and 2 to 5 ounces of aniline brown, with or without 1/2 to 1 ounce of aniline violet, in 150 ounces of alcohol.
Copper Varnishes. These two are for polished objects:
I. One hundred and ten parts of sandarac and 30 parts of rosin, dissolved in sufficient quantity of alcohol; 5 parts of glycerine are to be added.
II.
Sandarac 10 parts
Rosin 3 parts
Glycerine 1/2 part
Alcohol, a sufficient quantity.
Dissolve the two rosins in sufficient alcohol and add the glycerine.
Decorative Metal Varnishes.
I II III IV
Per Per Per Per
Cent Cent Cent Cent
Seedlac 11.5 .. .. ..
Amber 7.6 .. .. 13.5
Gamboge 7.6 .. .. ..
Dragon's blood 0.18 .. .. ..
Saffron 0.16 .. .. ..
Sandarac .. 11.2 15.9 16.6
Mastic .. 6.5 14.0 3.4
Elemi .. 3.3 .. ..
Venice turpentine .. .. 1.0 3.4
Camphor .. 1.5 .. ..
Aloe .. .. .. 7.0
Alcohol 72.96 77.5 66.1 63.2
As will be seen, only natural colors are used. The so-called "gold lacquer" is composed as follows: Sandarac, 6.25 parts; mastic, 3 parts; shellac, 12.5 parts; Venice turpentine, 2.5 parts; aloe, 0.75 parts; gamboge, 3 parts; alcohol, 72 parts. The solution is filtered. Applied in a thin coating this varnish shows a handsome golden shade. Other metal varnishes have the following composition:
V VI VII
Per Per Per
Cent Cent Cent
Shellac 17.5 .. 18.0
Yellow acaroid gum 13.1 25.0 ..
Manila .. 8.0 9.0
Alcohol 69.4 67.0 63.0
[727]
VARNISHES
Gold Varnish.
I. A good gold varnish for coating moldings which produces great brilliancy is prepared as follows: Dissolve 3 pounds of shellac in 30 quarts of alcohol, 5 pounds of mastic in 5 quarts of alcohol, 3 pounds of sandarac in 5 quarts of alcohol, 5 pounds of gamboge in 5 quarts of alcohol, 1 pound of dragon's blood in 1 quart of alcohol, 3 pounds of saunders in 5 quarts of alcohol, 3 pounds of turpentine in 3 quarts of alcohol. After all the ingredients have been dissolved separately in the given quantity of absolute alcohol and filtered, the solutions are mixed at a moderate heat.
II. A varnish which will give a splendid luster, and any gold color from deep red to golden yellow, is prepared by taking 50 ounces pale shellac, 15 pounds Florentine lake (precipitated from cochineal or redwood decoction by alum onto strach, kaolin, or gypsum), 25 ounces of sandalwood, and 8 ounces of dragon's blood. These in fine powder are dissolved on the water bath, in 500 ounces rectified spirit. The spirit must boil and remain, with occasional shaking, for 2 to 3 hours on the bath. Then cool and decant. In the meantime heat in another flask on the bath 30 ounces of gamboge in 500 ounces of the same spirit. The two liquids are mixed until the right color needed for the particular purpose in hand is obtained. Dilute with spirit if too thick. The addition of a little picric acid gives a greenish-yellow bronze but makes the varnish very liable to explode. These varnishes are applied to gently warmed surfaces with a soft bristle brush.
Gold Varnish for Tin. This is obtained in the following manner: Spread out 5 parts, by weight, of finely powdered crystallized copper acetate in a warm spot, allowing it to lie for some time; then grind the powder, which will have acquired a light-brown shade, with oil of turpentine and add, with stirring, 15 parts, by weight, of fat copal varnish heated to 140º F. When the copper acetate has dissolved (in about 1/4 hour), the mass is filled in a bottle and allowed to stand warm, for several days, shaking frequently. The gold varnish is then ready for use. Coat the articles uniformly with it, and heat in a drying chamber, whereupon, according to the degree of temperature, varying colorations are obtained, changing from green to yellow, then golden yellow, and finally orange to brown. When good copal varnish is employed, the varnish will adhere very firmly, so that the article can be pressed without damage.
Iron Varnishes.
I. A varnish obtained by dissolving wax in turpentine is useful. It gives a fairly hard coat, but has the drawback of filling up fine grooves, and so injuring the appearance of many metal ornaments.
II. Shellac, 15 pounds; Siam benjamin, 13 pounds; alcohol, 80 pounds; formylchloride, 20 pounds.
III. Sierra Leone copal, 6 pounds; dammar, 18 pounds; oleic acid, 3 pounds; alcohol, 40 pounds; oil of turpentine, 20 pounds; formylchloride, 15 pounds. The formylchloride not only effects the rapid drying necessary to prevent the varnish gravitating into hollows, but enables the alcohol to make a perfect solution of the rosin. The varnishes are excessively volatile, and must be stored accordingly.
Stove Varnishes.
Shellac 12 parts
Manila copal 14 parts
Rosin 12 parts
Gallipot 2 parts
Benzoin 1 part
Lampblack 5 parts
Nigrosin, spirit-soluble 1 1/2 parts
Alcohol 250 parts
Tin Varnishes.
I. For Tin Boxes. In 75 parts of alcohol dissolve 15 parts of shellac, 2 parts of Venice turpentine, and 8 parts of sandarac.
II. For Trays and Other Tinware. The ground is prepared by adding to the white lead the tinting colors ground in good rubbing varnish and half oil of turpentine. For drier an admixture of "terebine" is recommended. With this lean and dull paint, coat the tins 2 or 3 times and blend. Next, grain with water or vinegar glaze, and varnish with pure Zanzibar copal varnish, or finest amber table-top varnish. There are other tried methods for varnishing tin, which are applicable for new goods, manufactured in large quantities, while they are less advantageous for the restoration of old, repeatedly used articles.
VARNISH SUBSTITUTES.
A substitute for varnish is produced by adding to 100 parts of casein 10 to 25 parts of a 1 to 10 per cent soap solution and then 20 to 25 parts of slaked lime. . The mixture is carefully kneaded until a perfectly homogeneous mass results. Then gradually add 25 to 40 parts of turpentine oil and sufficient
[728]
VARNISHES - VETERINARY FORMULAS
water for the mass to assume the consistency of varnish. If it is desired to preserve it for some time a little ammonia is added so that the casein lime does not separate. The surrogate is considerably cheaper than varnish and dries so quickly that paint ground with it may be applied twice in quick succession.
Zapon Varnishes. In the case of many articles which have been colored mechanically or by the battery, particularly with large pieces, an opaque varnish is used as a protection against atmospheric influences. The so-called brassoline, of a brown color, negroline, black, and zapon. which is colorless, are employed, according to the color of the article. The last-named varnish is most commonly used, and gives a fine and durable coating, insoluble in almost all liquids which would come into consideration here, except that it will wash off in soap and water. Zapon varnish is a solution of collodion cotton and camphor in amyl acetate and amyl alcohol, and was formerly used to preserve old manuscripts and legal documents. In the process of zaponizing, the article is slightly warmed and immersed in the varnish, or the latter is applied with a brush. The solution is very durable, and has the advantage that after drying it will not show edges, rings, or spots. Zapon varnish which has become too thick must be diluted, and the brushes must be kept from becoming dry. If it is desired to give an especially warm tone, the article is treated with brushes which have been drawn over beeswax or mineral wax.
For the production of zapon or celluloid varnish, pour 20 parts of acetone over 2 parts of colorless celluloid waste, allowing it to stand for several days in a closed vessel, stirring frequently until the whole has dissolved into a clear, thick mass. Admix 78 parts of amyl acetate and clarify the zapon varnish by allowing it to settle for weeks.
VARNISH, HOW TO POUR OUT:
See Castor Oil.
VARNISHES, INSULATING:
See Insulation.
VARNISHES, PHOTOGRAPHIC RETOUCHING:
See Photography.
VARNISH REMOVERS:
See Cleaning Preparations and Methods.
VASELINE STAINS, TO REMOVE FROM CLOTHING:
See Cleaning Preparations and Methods.
VASOLIMENTUM.
This unguent is of two kinds, liquid and semi-solid. The former is prepared by mixing 500 parts of olein, 250 parts of alcoholic ammonia, and 1,000 parts of liquid paraffine, the whole being warmed until completely dissolved, and any loss in weight made up by addition of spirit. The semi-solid preparation is made of the same ingredients, except the paraffine salve is substituted for the liquid. The product is used as a basis for ointments in place of vasogene, and can be incorporated with a number of medicaments, such as 10 per cent of naphthol, 20 per cent of guaiacol, 25 per cent of juniper tar, 5 per cent of thiol, 6 per cent of iodine, 5 per cent of creosote, 10 per cent of ichthyol, 5 per cent of creolin, 2 per cent of menthol, etc.
VAT ENAMELS AND VARNISHES:
See Varnishes.
VEGETABLES, TESTS FOR CANNED:
See Foods.
VEGETABLE PARCHMENT:
See Parchment.
VICHY:
See Waters.
VICHY SALT:
See Salts (Effervescent).
Veterinary Formulas
FOR BIRDS:
Asthma in Canaries.
Tincture capsicum 5 drachms
Spirits chloroform 90 minims
Iron citrate, soluble 45 grains
Fennel water 3 1/2 ounces
Give a few drops on lump of sugar in the cage once daily.
Colas.
Tincture ferri perchloride 1 drachm
Acid hydrochloric, dil. 1/2 drachm
Glycerine l 1/2 drachms
Aqua camphor, q.s. 1 ounce
Use 3 to 6 drops in drinking water.
Ointment for Healing.
Peru balsam 60 grains
Cola cream 1 ounce
Apply.
[729]
VETERINARY FORMULAS
Constipation in Birds.
F.E. senna 2 drachms
Syrup manna 1 ounce
Fennel water, q. s. 4 ounces
Give a few drops on sugar in cage once daily.
Diarrhoea.
Tincture iron chloride 2 drachms
Paregoric 2 drachms
Caraway water 3 1/2 ounces
Give few drops on lump of sugar once daily.
Mocking-Bird Food.
Crackers 8 ounces
Corn 9 ounces
Rice 2 ounces
Hemp seed 1 ounce
Capsicum 10 grains
Mix and reduce to a coarse powder.
Foods for Red Birds.
Sunflower seed 8 ounces
Hemp seed 16 ounces
Canary seed 10 ounces
Cracked wheat 8 ounces
Unshelled rice 6 ounces
Mix and grind to a coarse powder.
Canary-Bird Food.
Yolk of egg (dry) 2 ounces
Poppy heads (powdered) 1 ounce
Cuttlefish bone (powdered) 1 ounce
Sugar 2 ounces
Powdered crackers 8 ounces
Bird Tonic.
Powdered capsicum 20 grains
Powdered gentian 1 drachm
Ferri peroxide 1/2 ounce
Powdered sugar 1/2 ounce
Syrup, q. s.
Put a piece size of pea in cage daily.
Tonic.
I.
Tincture cinchona 1/2 drachm
Tincture iron 2 drops
Glycerine 1 drachm
Caraway water 1 ounce
Put a few drops on lump of sugar in cage daily.
II.
Compound tincture cinchona 2 drachms
Compound tincture gentian 2 drachms
Syrup orange 1 ounce
Simple elixir 2 1/2 ounces
Put a few drops on lump of sugar in the cage daily.
Antiseptic Wash for Cage Birds.
Chinosol, F 2 drachms
Sugar (burnt) 20 minims
Aqua cinnamon 4 ounces
Aqua 20 ounces
Add 1 or 2 teaspoonfuls to the bath water and allow the birds to use it, when it will quickly destroy all parasites or germs in the feathers. To wash out the cages, use a mixture of 1 tablespoonful in a pint of hot water.
Mixed Bird Seed.
Sicily canary 10 ounces
German rape 2 ounces
Russian hemp 1 ounce
German millet 3 ounces
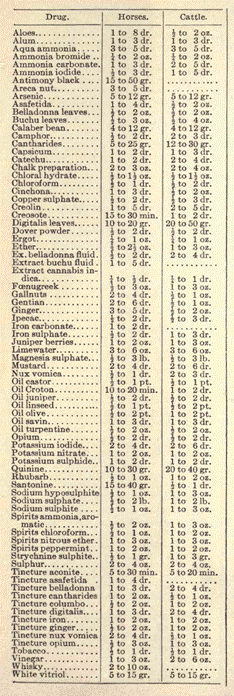
FOR HORSES AND CATTLE:
Blistering. Tincture cantharides, 1 ounce; camphorated oil, 1/2 ounce. Apply a portion with friction 3 times a day until a blister shows. As it subsides apply again.
Horse Colic Remedy.
I. In making a horse colic remedy containing tincture of opium, ether and chloroform, to be given in tablespoonful doses, apportion the ingredients about equally, and mix the dose with a pint of water.
Other formulas are:
II.
Chloroform anodyne 1 ounce
Spirit of nitrous ether 2 ounces
Linseed oil 13 ounces
Give in one dose and repeat in an hour if necessary.
Condition Powders.
I. Sulphur, 2 pounds; Glauber salts, 1 pound; black antimony, 1/2 pound; powdered bloodroot, 4 ounces; copperas, 1/2 pound; rosin, 1/2 pound; asafetida, 2 ounces; saltpeter 1/2 pound. Powder and mix well.
II. Gentian, 4 ounces; potassium nitrate, 1 ounce; sulphur, 4 ounces; ginger (African), 4 ounces; antimony, 4 ounces; rosin, 2 ounces; Foenugreek, 2 ounces; capsicum, 2 ounces; serpentaria, 2 ounces; sodium sulphate, 9 ounces; flaxseed meal, 16 ounces. All ingredients in fine powder. Dose: 1 tablespoonful in feed twice a day.
Veterinary Dose Table. For a colt 1 month old give 1/24 of the full dose; 3 months old, 1/12; 6 months old, 1/6; 1 year old, 1/3; 2 years old, 1/2; 3 years old, 3/4. Fluids for cattle usually the same dose as for the horse. Solids for cattle usually 1 1/2times the dose for the horse.
[730]
VETERINARY FORMULAS
Astringent.
I.
Opium 12 grains
Camphor 1/2 drachm
Catechu 1 drachm
One dose.
II.
Opium 12 grains
Camphor 1 drachm
Ginger 2 drachms
Castile soap 2 drachms
Anise 3 drachms
Licorice 2 drachms
Contracted Hoof or Sore Feet.
I.
Equal parts.
Lard
Yellow wax.
Linseed oil
Venice turpentine
Tar
Apply to the edge of the hair once a day.
II.
Rosin 4 ounces
Lard 8 ounces
Melt and add
Powdered vertigris 1 ounce
Stir well; when partly cool add
Turpentine 2 ounces
Apply to hoof about 1 inch down from the hair.
Cough.
I.
Sodii bromide 180 grains
Creosote water 2 ounces
Fennel water 4 ounces
Half tablespoonful 4 times daily.
II.
Ammonia bromide 180 grains
Fennel water 4 ounces
Syrup licorice 4 ounces
Teaspoonful 4 times daily
Cow Powder.
Powdered catechu 60 grains
Powdered ginger 240 grains
Powdered gentian 240 grains
Powdered opium 30 grains
CUTS, WOUNDS, SORES.
I. Tincture opium, 2 ounces; tannin, 1/4 ounce.
II. Tincture aloes, 1 ounce; tincture of myrrh, 1/2 ounce; tincture of opium, 1/2 ounce; water, 4 ounces. Apply night and morning.
III. Lard, 4 ounces; beeswax, 4 ounces; rosin, 2 ounces; carbolic acid, 1/4 ounce.
Diarrhoea.
I.
Opium 15 grains
Peppermint 1/2 ounce
Linseed meal 1 ounce
Give half in morning and remainder in evening in a pint of warm water.
[731]
VETERINARY FORMULAS
II.
Prepared chalk 6 ounces
Catechu 3 ounces
Opium 1 1/2 ounces
Ginger 3 ounces
Gentian 3 ounces
One powder 3 times a day in half a pint of warm water. One-sixth of dose for calves.
Diuretic Ball.
I.
Oil juniper 1/2 drachm
Rosin 2 drachms
Saltpeter 2 drachms
Camphor 1/2 drachm
Castile soap 1 ounce
Flaxseed meal 1 ounce
Make 1 pill.
II.
Rosin 90 grains
Potassium nitrate 90 grains
Po buchu leaves 45 grains
Dose: 1 twice a day.
Drying Drink.
Powdered alum 6 ounces
Armenian bole 2 ounces
Powdered juniper berries 1/2 ounces
Once daily in 1 quart of warm gruel.
Epizooty or Pinkeye.
Sublimed sulphur 1/2 ounce
Epsom salt 1 ounce
Charcoal 1/2 ounce
Extract licorice 1 ounce
Fever.
I.
Salicylic acid 3/4 ounce
Sodium bicarbonate 1/2 ounce
Magnesium sulphate. 10 ounces
Give half in quart of warm bran water at night.
II.
Spirits niter 3 ounces
Tincture aconite 2 drachms
Fluid extract belladonna 1/2 ounce
Nitrate potash 2 ounces
Muriate ammonia 2 ounces
Water, q. s 1 quart
Dose: Teaspoonful every 2 or 3 hours till better.
Heaves.
I. Balsam copaiba, 1 ounce; spirits of turpentine, 2 ounces; balsam fir, 1 ounce; cider vinegar, 16 ounces.
Tablespoonful once a day.
II. Saltpeter, 1 ounce; indigo, 1/2 ounce; rain or distilled water, 4 pints. Dose: 1 pint twice a day.
Hide Bound.
Elecampane 2 ounces
Licorice root 2 ounces
Foenugreek 2 ounces
Rosin 2 ounces
Copperas 1/2 ounce
Ginger 2 drachms
Gentian 1 drachm
Saltpeter 1 drachm
Valerian 1 drachm
Linseed meal 3 ounces
Sublimed sulphur 1 ounce
Black antimony 4 drachms
Tablespoonful twice a day.
HORSE EMBROCATIONS AND LINIMENTS.
I.
Camphor 1 ounce
Acetic acid 15 ounces
Alcohol 18 ounces
Oil turpentine 51 ounces
Eggs 6
Distilled witch hazel 45 ounces
II.
Iodine 50 grains
Pot iodide 125 grains
Soap liniment 6 ounces
INFLUENZA.
I.
Ammonia muriate 1 1/2 ounces
Gum camphor 1/2 ounce
Pot chloride 1 ounce
Extract licorice, powdered 2 ounces
Molasses, q.s.
Make a mass. Dose: Tablespoonful in form of pill night and morning.
II.
Ammonium chloride 30 parts
Potassium nitrate 30 parts
Potassium sulphate in
little crystals 100 parts
Licorice powder 65 parts
Mix. Dose: A tablespoonful, in a warm mash, 3 times daily.
INFLAMMATION OF THE UDDER.
I.
Salicylic acid 40 grains
Mercurial ointment 1 ounce
Liniment of camphor 3 1/4 ounces
Apply and rub the udder carefully twice a day.
II.
Belladonna root 1 drachm
Oil turpentine 1 ounce
Camphor 1 drachm
Solution green soap, q.s. 6 ounces Mix and make a liniment. Bathe the udder several times with hot water. Dry and apply above liniment.
MANGE.
Sulphur is a specific for mange; the trouble consists in its application, "The
[732]
VETERINARY FORMULAS
old-fashioned lotion of train oil and black sulphur serves well enough, but for stabled animals something is wanted which will effectually destroy the parasites in harness and saddlery without injury to those expensive materials. The creosote emulsions and coal-tar derivatives generally are fatal to the sarcopts if brought into actual contact, but a harness pad with ridges of accumulated grease is a sufficient retreat for a few pregnant females during a perfunctory disinfection, and but a few days will be needed to reproduce a new and vigorous stock. A cheap and efficient application can be made by boiling together flowers of sulphur and calcis hydras in the proportion of 4 parts of the former to 1 of the latter, and 100 of water, for half an hour. It should be applied warm, or immediately after washing with soft soap.
Milk Powder for Cows. For increasing the flow of milk, in cows, Hager recommends the following mixture:
Potassium nitrate 1 part
Alum 1 part
Sublimed sulphur 1 part
Prepared chalk 1 part
White bole 2 parts
Red clover 5 parts
Anise 10 parts
Fennel 10 parts
Salt 10 parts
All should be in tolerably fine powder and should be well mixed. The directions are to give 1 or 2 handfuls with the morning feed.
LAXATIVES.
I.
Aloes 1 drachm
Soap 12 drachms
Caraway 4 drachms
Ginger 4 drachms
Treacle, q. s.
Make 4 balls. Dose: 1 daily.
II.
Rochelle salts 2 ounces
Aloes, powdered 150 grains
Linseed meal 150 grains
One dose, given in warm water.
Lice.
Crude oil 1 ounce
Oil tar 1 ounce
Oil cedar 1 drachm
Cottonseed oil 5 ounces
Apply to parts.
DOMESTIC PETS.
The sarcoptic itch of the dog, as well as that of the cat, is transmissible to man.
The Tinea tonsurans, the so-called barbers' itch, due to a trychophyton, and affecting both the dog and cat, is highly contagious to man. Favus, Tinea favos, caused by achorion schoenleini, of both animals, is readily transmissible to human beings. The dog carries in his intestines many kinds of taenia (tapeworm), among them Taenia echinococcus, the eggs of which cause hydatic cysts. Hydatic cysts occur in persons who are always surrounded with dogs, or in constant contact with them.
Aviar diphtheria (i.e, the diphtheria of birds), caused by at least two microbes (bacillus of Klebs-Loeffler and bacillus coli), may easily be transmitted to man and cause in him symptoms analogous to those of true diphtheritic angina.
Parrots are subject to an infectious enteritis which may be communicated to human beings, giving rise to the socalled psittacosis (from the Greek, psitta, a parrot), of which there have been a number of epidemics in France. It is determined by the bacillus of Nocard.
Human tuberculosis is certainly transmitted to dogs, cats, and birds. Cadiot, Gibert, Roger, Benjamin, Petit, and Basset, as well as other observers, cite cases where dogs, cats, and parrots, presenting all the lesions of tuberculosis, were shown to have contracted it from contact with human beings; while there are no recorded cases, there can scarcely be a natural doubt that man may, in a similar manner, become attainted through them, and that their tuberculosis constitutes an actual danger to man.
Need we recall here the extraordinary facility with which hydrophobia is communicated to man through the dog, cat, etc.?
We may, therefore, conclude that we should not permit these animals to take up so much space in our apartments, nor should they be petted and caressed either by adults or children in the reckless manner common in many households. The disgusting habit of teaching animals to take bits of food, lumps of sugar, etc., from between the lips of members of the family is also to be shunned.
Finally, any or all of them should be banished from the house the moment that they display certain morbid symptoms. Besides, in certain cases, there should be a rigid prophylaxis against certain diseases as echiuococcus, for instance.
Worms. In cats and dogs, round worms, of which ascaris mystax is the
[733]
VETERINARY FORMULAS
most common in cats, are found chiefly in young animals. This worm has hirsute appendages somewhat resembling a mustache. To treat an animal infected with such "guests," the patient should be made to fast for 24 hours. For a small kitten 1/2 grain of santonin, up to a grain or two for large cats, followed in an hour by a dose of castor oil, is recommended. To avoid spilling the oil on the animal's coat the "doctor" should have it heated and whipped with warm milk. Another way to get cats to take it is to smear it on the bottoms of their front feet, when they will lick it off. Areca nut, freshly ground by the druggist himself and administered in liberal doses, say 30 to 60 grains, will usually drive out any worms in the alimentary canal.
It is important that animals successfully treated for worms once should undergo the treatment a second or third time, as all the parasites may not have been killed or removed the first time, or their progeny may have developed in the field vacated by the parents.
The following is an effective formula:
German wormseed, powdered 1 drachm
Fluid extract of spigelia 3 drachms
Fluid extract of senna 1 drachm
Fluid extract of valerian 1 drachm
Syrup of buckthorn 2 ounces
Dose: From 1/2 to 1 teaspoonful night and morning.
Foot Itch. The itch that affects the feet of poultry is contagious in a most insidious way. The various birds of a poultry yard in which the disease is prevalent, rarely contract it until after a comparatively long period of exposure, but sooner or later every bird will contract it. One infected bird is enough to infect a whole yard full, and once infected, it is exceedingly difficult to get rid of. The disease, however, affects birds only.
The treatment is simple. Having softened the feet by keeping them for some minutes in tepid water, the scabs that cover them are carefully detached, avoiding, as far as possible, causing them to bleed, and taking the precaution of throwing every scab into the fire. The feet are then carefully dried, with a bit of soft cotton material, which should afterwards be burned; then the entire surface is covered with ointment (Unguentum sulphuris kalinum). An alcoholic solution of Canada balsam is preferred by some.
Protect the ointment by a proper appliance, and allow it to remain in contact 2 or 3 days. At the end of this time remove the applications and wash off with tepid suds. The bird will generally be found cured, but if not, repeat the treatment removing the remaining scabs, which will be found soft enough without resorting to soaking in tepid water, and apply the ointment directly.
There is another method of treatment that has been found successful, which not only cures the infected birds but prevents the infection of others. It is simply providing a sand bath for the birds, under a little shed, where they can indulge themselves in rolling and scratching, the bath being composed of equal parts fine sand, charcoal in fine powder, ashes, and flowers of sulphur, sifted together. .The bath should be renewed every week. In the course of a few weeks the cure is complete.
Foods.
I.
Powdered egg shell or phosphate
of lime 4 ounces
Iron sulphate 4 ounces
Powdered capsicum 4 ounces
Powdered Foenugreek 2 ounces
Powdered black pepper 1 ounce
Silver sand 2 ounces
Powdered lentils 6 ounces
A tablespoonful to be mixed with sufficient feed for 20 hens.
II.
Oyster shell, ground 5 ounces
Magnesia 1 ounce
Calcium carbonate 3 ounces
Bone, ground 1 1/2 ounces
Mustard bran 1 1/2 ounces
Capsicum 1 ounce
Powders.
I.
Cayenne pepper 2 parts
Allspice 4 parts
Ginger 6 parts
Powder and mix well together. A teaspoonful to be mixed with every pound of food, and fed 2 or 3 times a week. Also feed fresh meat, finely chopped.
II.
Powdered egg shells 4 parts
Powdered capsicum 4 parts
Sulphate of iron 4 parts
Powdered Foenugreek 2 parts
Powdered black pepper 1 part
Sand 2 parts
Powdered dog biscuit 6 parts
A tablespoonful to be mixed with sufficient meal or porridge to feed 20
hens.
[734]
VETERINARY FORMULAS VINEGAR
Lice Powders.
I.
Sulphur 4 ounces
Tobacco dust 6 ounces
Cedar oil 1/4 ounce
White hellebore 4 ounces
Crude naphthol 1 ounce
Powdered chalk, q. s. 2 pounds
II.
Sulphur 1 ounce
Carbolic acid 1/4 ounce
Crude naphthol 1 ounce
Powdered chalk 1 pound
Roup or Gapes. Roup in poultry is caused by the presence of parasites or entozoa in the windpipe. Young birds are most commonly affected. The best method of treatment is to expose the affected bird to the fumes of heated carbolic acid until on the point of suffocation. The bird may be placed in a box with a hot brick, and carbolic acid placed thereon. The fowls soon recover from the incipient suffocation, and are almost always freed from the disease. Care must be taken to burn the parasites coughed out, and the bodies of any birds which may die of the disease. The following powders for the treatment of "roup" in poultry have been recommended:
I.
Potassium chlorate 1 ounce
Powdered cubebs 1 ounce
Powdered anise 1/2 ounce
Powdered licorice l 1/2 ounces
Mix a teaspoonful with the food for 20 hens.
II.
Ammonium chloride 1 ounce
Black antimony 1/4 ounce
Powdered anise 1/2 ounce
Powdered squill 1/4 ounce
Powdered licorice 2 ounces
Mix and use in the foregoing.
FOR SHEEP:
Dips. For the prevention of "scab" in sheep, which results from the burrowing of an acarus or the destruction of the parasite when present, various preparations of a somewhat similar character are used. The following formulas for sheep dips are recommended by the United States Department of Agriculture:
I.
Soap 1 pound
Crude carbolic acid 1 pint
Water 50 gallons
Dissolve the soap in a gallon or more of boiling water, add the acid, and stir thoroughly.
II.
Fresh skimmed milk 1 gallon
Kerosene 2 gallons
Churn together until emulsified, or mix and put into the mixture a force pump and direct the stream from the pump back into the mixture. The emulsification will take place more rapidly if the milk be added while boiling hot.
Use 1 gallon of this emulsion to each 10 gallons of water required.
Constipation.
I.
Green soap 150 grains
Linseed oil 1 1/2 ounces
Water 15 ounces
Give 1/6 every 1/2 hour till action takes place.
II.
Calomel 1 1/2 grains
Sugar 15 grains
One dose.
Loss of Appetite.
Sodium sulphate, dried 90 grains
Sodium bicarbonate 30 grains
Rhubarb 30 grains
Calamus 90 grains
Form the mass into 6 pills. Give one twice daily.
Inflammation of the Eyes.
Zinc sulphate 20 grains
Mucilage quince seed 4 ounces
Distilled water 4 ounces
Bathe eyes twice daily.
Vinegar
I. Into a hogshead with a large bunghole put 1,500 parts, by weight, of honey, 125 parts of carob-pods, cut into pieces, 50 parts of powdered red or white potassium bitartrate, 125 parts of powdered tartaric acid, 2,000 parts of raisin stems, 400 parts of the best brewers' yeast, or 500 of leaven rubbed up in water; add 16,000 parts of triple vinegar and 34,000 parts of 40 per cent spirit, containing no fusel oil. Stir all vigorously together; fill up the hogshead with hot water (100º F.), close the bunghole with gauze to keep out insects, and let the contents of the cask stand for from 4 to 6 weeks or until they have turned to vinegar. The temperature of the room should be from 77º to 88º F.
Draw off half the vinegar, and fill the hogshead up again with 15 parts of soft water and 1 part of spirit (40 per cent). Do this 4 times, then draw off all the vinegar and begin the first process over again. This method of making vinegar is suitable for households and small dealers, but would not suffice for whole-
[735]
VINEGAR
sale manufacturers, since it would take too long to produce any large amount.
II. Put into an upright wine cask open at the top, 14,000 parts, by weight, of lukewarm water, 2,333 parts of 60 per cent alcohol, 500 parts of brown sugar, 125 parts of powdered red or white potassium bitartrate, 250 parts of good brewers' yeast, or 125 parts of leaven,
1,125 parts of triple vinegar, and stir until the substances are dissolved. Lay a cloth and a perforated cover over the cask and let it stand in a temperature of 72º to 77º F. from 4 to 6 weeks; then draw off the vinegar. The thick deposit at the bottom, the "mother of vinegar," so called, can be used in making more vinegar. Pour over it the same quantities of water and alcohol used at first; but after the vinegar has been drawn off twice, half the first quantity of sugar and potassium bitartrate, and the whole quantity of yeast, must be added. This makes excellent vinegar.
III. A good strong vinegar for household use may be made from apple or pear peelings. Put the peelings in a stone jar (not glazed with lead) or in a cask, and pour over them water and a little vinegar, fermented beer, soured wine, or beet juice. Stir well, cover with a linen cloth and leave in a warm room. The vinegar will be ready in 2 or 3 weeks.
IV. Two wooden casks of any desired size, with light covers, are provided. They may be called A and B. A is filled with vinegar, a tenth part of this is poured off into B, and an equal amount of fermented beer, wine, or any other sweet or vinous liquid, or a mixture of
1,125 parts, by weight, of alcohol, 11,500 to 14,000 parts of water, and 1,125 parts of beet juice, put into A.
When vinegar is needed, it is drawn out of B, an equal quantity is poured from A into B and the same quantity of vinegar-making liquids put into A. In this way vinegar is constantly being made and the process may go on for years, provided that the casks are large enough so that not more than a tenth of the contents of A is used in a week. If too much is used, so that the vinegar in the first cask becomes weak, the course of the vinegar making is disturbed for a long time, and this fact, whose importance has not been understood, prevents this method in its essential principles the best from being employed on a large scale. The surplus in A acts as a fermentative.
Aromatic Vinegar.
I. Sixteen ounces glacial acetic acid, 40 drops oil of cloves, 40 drops oil of rosemary, 40 drops oil of bergamot, 16 drops oil of neroli, 30 drops oil of lavender, 1 drachm benzoic acid, 1/2 ounce camphor, 30 to 40 drops compound tincture of lavender, 3 ounces spirit of wine. Dissolve the oils, the benzoic acid, and the camphor in the spirit of wine, mix with acetic acid and shake until bright, lastly adding the tincture of lavender to color.
II. Dried leaves of rosemary, rue, wormwood, sage, mint, and lavender flowers, each 1/2 ounce; bruised nutmegs, cloves, angelica root, and camphor, each 1/4 of an ounce; rectified alcohol, 4 ounces; concentrated acetic acid, 16 ounces. Macerate the materials for a day in the alcohol; then add the acid and digest for 1 week longer at a temperature of 490º F. Finally press put the now aromatised acid and filter it.
Cider Vinegar. By "artificial vinegar" is meant vinegar made by the quick method with beech wood shavings. This cannot be carried out with any economy on a small scale, and requires a plant. A modification of the regular plan is as follows: Remove the head from a good tight whisky barrel, and put in a wooden faucet near the bottom. Fill the barrel with corn cobs and lay an empty coffee sack over them. Moisten the cobs by sprinkling them with some good, strong, natural vinegar, and let them soak for a few hours. After the lapse of 2 or 3 hours draw off the vinegar and again moisten the cobs, repeating this until they are rendered sour throughout, adding each time 1 quart of high wines to the vinegar before throwing it back on the cobs. This prevents the vinegar from becoming flat, by the absorption of its acetic acid by the cobs. Mix a gallon of molasses with a gallon of high wine and 14 gallons of water and pour it on the cobs. Soak for 8 hours, then draw off and pour on the cobs again. Repeat this twice daily, until the vinegar becomes sour enough to suit. By having a battery of barrels, say 4 barrels prepared as above, the manufacture may be made remunerative, especially if the residue of sugar casks in place of molasses, and the remnants of ale, etc., from the bar-rooms around town are used. All sugar-containing fruit may be utilized for vinegar making.
VINEGAR, TESTS FOR:
See Foods.
VINEGAR, TOILET:
See Cosmetics.
[736]
WARTS - WATCHMAKERS' FORMULAS
VIOLET AMMONIA:
See Cosmetics.
VIOLET WATER:
See Perfumes.
VIOLIN ROSIN:
See Rosin.
VIOLIN VARNISH:
See Varnishes.
VISCOSE:
See Celluloid.
VOICE LOZENGES:
See Confectionery.
VULCANIZATION OF RUBBER:
See Rubber.
WAGON GREASE:
See Lubricants.
WALLS, DAMP:
See Household Formulas.
WALL AND WALL PAPER CLEANERS:
See Cleaning Preparations and Methods, also Household Formulas.
WALL PAPER DYES:
See Dyes.
WALL PAPER PASTE:
See Adhesives.
WALL PAPER, REMOVAL OF:
See Household Formulas.
WALL WATERPROOFING:
See Waterproofing and Household Formulas.
WALL PRIMING:
See Paints.
WALNUT:
See Wood.
WARMING BOTTLE:
See Bottles.
WARPING, PREVENTION OF:
See Wood.
Warts
Wart Cure. The following is especially useful in cases where the warts
are very numerous:
I.
Chloral hydrate 1 part
Acetic acid 1 part
Salicylic acid 4 parts
Sulphuric ether 4 parts
Collodion 15 parts
Mix. Directions: Every morning apply the foregoing to the warts, painting one coat on another. Should the mass fall off without taking the warts with it, repeat the operation. Take, internally 10 grains of burnt magnesia daily.
II.
Sulphur 10 parts
Acetic acid 5 parts
Glycerine 5 parts
Keep the warts covered with this mixture.
WASHING FLUIDS AND POWDERS:
See Laundry Preparations.
WASTE, PHOTOGRAPHIC, ITS DISPOSITION:
See Photography.
WATCH-DIAL CEMENTS:
See Adhesives, under Jewelers' Cements.
WATCH GILDING:
See Plating.
Watchmakers' Formulas
WATCH MANUFACTURERS' ALLOYS.
Some very tenacious and hard alloys, for making the parts of watches which are not sensitive to magnetism, are as follows:
|
|
I |
II |
III |
IV |
V |
VI |
VII |
|
Platinum |
62.75 |
62.75 |
62.75 |
54.32 |
0.5 |
0.5 |
- |
|
Copper |
18 |
16.20 |
16.20 |
16 |
18.5 |
18.5 |
25 |
|
Nickel |
18 |
18 |
16.50 |
24.70 |
- |
2 |
1 |
|
Cadmium |
1.25 |
1.25 |
1.25 |
1.25 |
- |
- |
- |
|
Cobalt |
- |
- |
1.50 |
1.96 |
- |
- |
- |
|
Tungsten |
- |
1.80 |
1.80 |
1.77 |
- |
- |
- |
|
Palladium |
- |
- |
- |
- |
72 |
72 |
70 |
|
Silver |
- |
- |
- |
- |
6.5 |
7 |
4 |
|
Rhodium |
- |
- |
- |
- |
1 |
- |
- |
|
Gold |
- |
- |
- |
- |
1.5 |
- |
- |
A non-magnetic alloy for watch springs, wheels, etc.: Gold, 30 to 40 parts; palladium, 30 to 40 parts; copper, 10 to 20 parts; silver, 0.1 to 5 per cent; cobalt, 0.1 to 2.5 per cent; tungsten, 0.1 to 5 per cent; rhodium, 0.1 to 5 per cent; platinum, 0.1 to 5 per cent.
An Alloy for Watch Pinion Sockets. Gold, 31 parts; silver, 19 parts; copper, 39 parts; palladium, 1 part.
Replacing Rubies whose Settings have Deteriorated. Enlarge, with the squarer (steel brooch for enlarging holes), the hole of the old setting, and adjust it, with hard rubbing, to the extremity of a stem of pierced brass wire. Take the stem in an American nippers, and set the ruby at the extremity (the setting may be driven back by using a flat burnishing tool, very gently). Then take off with a cleaving file the part of the stem where the ruby is set, and diminish it to the thickness desired, by filing on the finger, or on cork. These operations finished’
[737]
WATCHMAKERS FORMULAS
a set stopper is obtained which now needs only to be solidly fixed at the suitable height, in the hole prepared.
To Straighten Bent Teeth. Bent teeth are straightened by means of the screwdriver used as a lever against the root of the adjacent teeth, and bent pivots may be held in the jaws of the pliers and the pinion bent with the fingers in the direction and to the extent required. For such a purpose, pliers having the jaws lined with brass are used so that the pivot is not bruised, and the bending has to be done with great care.
To Renew a Broken Barrel Tooth.
Frequently, in consequence of the breaking of a spring, a tooth of a barrel is broken. Sometimes it may only be bent, in which case the blade of a penknife may be used with care. If 2 or 3 successive teeth are lacking, the best way is to change the barrel, but a single tooth may be easily renewed in this way: Drill a hole through the thickness of the tooth, taking care not to penetrate the drum; then fit in a piece of metal tightly and give it, as well as possible, the correct form of the tooth. To assure solidity, solder it; then clean and round
the edges. Properly executed the repair will scarcely be noticed.
Heated Sawdust. Sawdust is known to have been employed from time immemorial by watchmakers and goldsmiths for the purpose of drying rinsed articles. The process of drying can be accelerated four-fold if the sawdust is heated before use. This must, however, be done with great caution and constant stirring.
To Repair a Dial, etc., with Enamel Applied Cold. There are two kinds of false enamel for application, when cold, to damaged dials. The first, a mixture of white rosin and white lead, melts like sealing wax, which it closely resembles. It is advisable when about to apply it to gently heat the dial and the blade of a knife, and with the knife cut the piece of enamel of the requisite size and lay it on the dial. The new enamel must project somewhat above the old. When cold the surface is leveled by scraping, and a shining surface is at once produced by holding at a little distance from the flame of a spirit lamp. It is necessary to be very careful in conducting this operation, as the least excess of heat will burn the enamel and turn it yellow. It is, however, preferable to the following although more difficult to apply, as it is harder and does not become dirty so soon. The second false enamel contains white lead mixed with melted white wax. It is applied like cement, neatly filling up the space and afterwards rubbing with tissue paper to produce a shining surface. If rubbed with a knife blade or other steel implement its surface will be discolored.
Lettering a Clock Dial. Painting Roman characters on a clock dial is not such a difficult task as might at first be imagined. If one has a set of drawing instruments and properly proportions the letters, it is really simple. The letters should be proportioned as follows: The breadth of an "I" and a space should equal 1/2 the breadth of an "X," that is, if the "X" is 1/2 inch broad, the "I" will be 3/16 inch broad and the space between letters 1/16 inch, thus making the "I" plus one space equal to 1/4 inch or half the breadth of an "X." The "V's" should be the same breadth as the "X's." After the letters have been laid off in pencil, outline them with a ruling pen and fill in with a small camel's-hair brush, using gloss black paint thinned to the proper consistency to work well in the ruling pen. Using the ruling pen to outline the letters gives sharp straight edges, which it would be impossible to obtain with a brush in the hands of an inexperienced person.
Verification of the Depthings. In the verge watches, the English watches, and those of analogous caliber, it is often difficult to verify the depthings, except by the touch. For this reason we often find the upper plate pierced over each depth. In the jeweled places, instead of perforating the upper plate, it suffices to deposit a drop of very limpid oil on the ruby, taking care that it does not scatter.
In this manner a lens is formed and one may readily distinguish the depthing.
To Make or Enlarge a Dial Hole. By wetting the graver or the file with spirit of turpentine, cracks may be avoided and the work will be accomplished much quicker.
To Repair a Repeating Clock Bell. When the bell is broken, whether short off or at a distance, file it away and pierce it, and after having sharpened a little the stem of the spring which remains, push
by force, in the hole just made, a thin piece of solder (pewter). The sound will not have changed in any appreciable manner.
A seconds pendulum of a regulator, which has no compensation for temperature will cause the clock to lose about
[738]
WATCHMAKERS' FORMULAS
1 second per day for each 3 degrees of increase in heat. A watch without a compensation balance will lose 6.11 seconds in 24 hours for each increase of 1º F. in heat.
To Remedy Worn Pinions. Turn the leaves or rollers so that the worn places upon them will be toward the arbor or shaft and fasten them in that position. If they are "rolling pinions," and they cannot be secured otherwise, a little soft solder should be used.
Watchmakers’ Oil.
I. Put some lead shavings into neat's foot oil, and allow to stand for some time, the longer the better. The lead neutralizes the acid, and the result is an oil that never corrodes or thickens.
II. Stir up for some time best olive oil with water kept at the boiling point; then after the two fluids have separated, decant the oil and shake up with a little freshly burned lime. Let the mixture stand for some weeks in a bottle exposed to the sunlight and air, but protected from wet and dirt. When filtered, the oil will be nearly colorless, perfectly limpid, and will never thicken or become rancid.
To Weaken a Balance Spring. A balance spring may need weakening; this is effected by grinding the spring thinner. Remove the spring from the collet and place it upon a piece of pegwood cut to fit the center coil. A piece of soft iron wire, flattened so as to pass freely between the coils and charged with a little powdered oilstone, will serve as a grinder, and with it the strength of the spring may soon be reduced. Operations will be confined to the center coil, for no other part of the spring will rest sufficiently against the wood to enable it to be ground, but this will generally suffice. The effect will be rather rapid; therefore care should be taken or the spring may be made too weak.
To Make a Clock Strike Correctly. Pry the plates apart on the striking side, slip the pivots of the upper wheels out, and having disconnected them from the train, turn them partly around and put them back. If still incorrect, repeat the experiment. A few efforts at most will
get them to work properly. The sound in cuckoo clocks is caused by a wire acting on a small bellows which is connected with two small pipes like organ pipes.
To Reblack Clock Hands. One coat of asphaltum varnish will make old rusty hands look as good as new, and will dry in a few minutes.
To Tighten a Ruby Pin. Set the ruby pin in asphaltum varnish. It will become hard in a few minutes and be much firmer and better than the gum shellac, generally used.
To Loosen a Rusty Screw in a Watch Movement. Put a little oil around the screw; heat the head lightly by means of a red-hot iron rod, applying the same for 2 or 3 minutes. The rusty screw may then be removed as easily as though it had just been put in.
Gilding Watch Movements. (See also Gilding.) In gilding watch movements, the greatest care must be observed with regard to cleanliness. The work is first to be placed into a weak solution of caustic potash for a few minutes, and then rinsed in cold water. The movements are now to be dipped into pickling acid (nitrous acid) for an instant, and then plunged immediately into cold water. After being finally rinsed in hot water, they may be placed in the gilding bath and allowed to remain therein until they have received the required coating. A few seconds will generally be sufficient, as this class of work does not require to be very strongly gilt. When gilt, the movements are to be rinsed in warm water, and scratch-brushed; they may then be returned to the bath, for an instant, to give them a good color. Lastly, rinse in hot water and place the movements in clean box sawdust. An economical mode of gilding watch movements is to employ a copper anode working from the solution, add 10 parts of cream of tartar and a corresponding quantity of elutriated chalk to obtain a pulp that can be put on with the brush. The gilding or silvering obtained in this manner is pretty, but of slight durability.
At the present time this method is only seldom employed, since the electroplating affords a means of producing gilding and silvering in a handsome and comparatively cheap manner, the metallic coating having to be but very thin. Gold and silver for this kind of work are used in the form of potassium cyanide of gold or potassium cyanide of silver solutions, it being a custom to copper the zinc articles previously by the aid of a battery, since the appearance will then be much handsomer than on zinc alone. Gilding or silvering with leaf metal is done by polishing the surface of the zinc bright and coating it with a very tough linseed-oil varnish diluted with 10 times the quantity of benzol. The metallic leaf is then laid on and polished with an agate.
[739]
WATER
WATCHMAKERS' CLEANING PREPARATIONS:
See Cleaning Preparations and Methods.
WATCH MOVEMENTS, PALLADIUM PLATING OF:
See Plating.
Water, Natural and Artificial
In making an artificial mineral water it must be remembered that it is seldom possible to reproduce the water by merely combining its chemical components. In other words, the analysis of the water cannot serve as a basis from which to prepare it, because even though all of the components were put together, many would be found insoluble, and others would form new chemical combinations, so that the result would differ widely from the mineral water imitated.
For example, carbonate of magnesia and carbonate of lime, which are important ingredients in most mineral waters, will not make a clear solution unless freshly precipitated. Hence, when these are to be reproduced in a mineral water it is customary to employ other substances which will dissolve at once, and which will, upon combining, produce these salts. The order in which the salts are added is also a very important matter, for by dissolving the salts separately and then carefully combining them, solutions may be effected which would be impossible were all the salts added together to the water in the portable fountain.
In this connection the following table will be found useful:
Group 1
|
Ammonium carbonate Ammonium chloride Sodium borate (borax) Potassium carbonate Potassium chloride Potassium nitrate Potassium sulphate Sodium bromide |
Sodium carbonate Sodium chloride Sodium fluoride Sodium iodide Sodium nitrate Sodium phosphate Sodium pyrophosphate Sodium silicate. Sodium sulphate |
Group 2
|
Lithium carbonate |
Group 3
|
Aluminum chloride Barium chloride Calcium bromide Calcium chloride Calcium nitrate |
Magnesium chloride Magnesium nitrate Strontium chloride. Lithium chloride |
Group 4
|
Magnesium sulfate |
Alum (potassa or phate. soda alum) |
Group 5
|
Lime carbonate Magnesium carbonate hydrate |
Lime sulphate precipitate
|
Group 6
|
Lithium carbonate Acid hydrochloric Acid sulphuric Iron chloride |
Iron pyrophosphate. Iron sulphate. Manganese chloride. Manganese sulphate |
Group 7
|
Sodium arseniate, or sodium sulphide, or acid hydrosulphuric |
Explanation of Groups. The explanation of the use of these groups is simple. When about to prepare an artificial mineral water, first ascertain from the formula which of the ingredients belong to group 1. These should be dissolved in water, and then be filtered and added to distilled water, and thoroughly agitated. Next the substance or substances belonging to group 2 should be dissolved in water, then filtered and added to the water, which should again be agitated.
And so the operation should proceed; whatever ingredients are required from each group should be taken in turn, a solution made, and this solution, after being filtered, should be separately added to the fountain, and the latter be well agitated before the following solution is added.
For groups 1, 3, and 4, the salts should be dissolved in 5 times their weight of boiling, or 10 times their weight of cold, water. For group 2 (lithium carbonate) the proportions should be 1 part of lithium carbonate to about 130 parts of cold or boiling water. The substances mentioned in group 5 are added to the portable fountain in their solid state, and dissolve best when freshly precipitated. As carbonic acid gas aids their solution, it is best to charge the fountain after they are added, and agitate thoroughly, blowing off the charge afterwards if necessary.
In group 5 the lithium carbonate is dissolved in the acids (see also group 2), the iron and manganese salts are dissolved in 5 parts of boiling, or 10 parts of cold, water, the solution quickly filtered, the acids added to it, and the whole mixture added to the fountain already charged with gas, the cap being quickly taken off, and the solution poured in. The iron and manganese salts easily oxidize and produce turbidity, therefore the atmospheric air should be carefully
[740]
WATER
blown off under high pressure several times while charging fountains. The substances mentioned in group 7 are never put into the fountain, except the arseniate of sodium in the case of Vichy water, which contains but a trifling amount of this compound.
Most of the solutions may be prepared beforehand and be used when required, thus saving considerable time.
Formulas for various waters will be given at the end of this article.
A question which arises in preparing mineral waters is: What is the best charging pressure? As a general rule, they are charged to a lower pressure than plain soda; good authorities even recommend charging certain mineral waters as low as 30 pounds pressure to the square inch, but this seems much too low a pressure for the dispensing counter. From 50 to 120 pounds pressure would be a good limit, while plain soda may be served out as high as 180 pounds. There must be enough pressure completely to empty the fountain, while enabling sufficient gas to be retained by the water to give it a thorough pungency. Moreover, a high pressure to the mineral water enables a druggist at a pinch, when he runs out of plain soda, to use his Vichy water, instead, with the syruped drinks. The taste of the Vichy is not very perceptible when covered by the syrup, and most customers will not notice it.
Apollinaris Water.
Sodium carbonate 2,835 grains
Sodium sulphate 335 grains
Sodium silicate 10 grains
Magnesium chloride 198 grains
Calcium chloride 40 grains
Potassa alum 57 grains
Magnesium carbonate hydrate 158 grains
Iron sulphate 21 grains
Hunyadi Water.
Magnesium sulphate 400 parts
Sodium sulphate 400 parts
Potassium sulphate 2 parts
Sodium chloride 31 parts
Sodium bicarbonate 12 parts
Water 1 quart
Lithia Water.
Lithium carbonate 120 grains
Sodium bicarbonate 1,100 grains
Carbonated water 10 gallons
For "still" lithia water, substitute lithium citrate for the carbonate in the above formula.
Seltzer Water. Hydrochloric acid (chemically pure), 2,520 grains; pure water, 40 ounces. Mix and add marble dust, 240 grains; carbonate of magnesium, 420 grains. Dissolve, and after 1 hour add bicarbonate of sodium, 2,540 grains. Dissolve, then add sufficient pure water to make 10 gallons. Filter and charge to 100 pounds pressure.
Vichy Water. The following formula, based on the analysis of Bauer-Struve, yields an imitation of
Vichy (Grande Grille).
Sodium iodide 0.016 parts
Sodium bromide 0.08 parts
Sodium phosphate 2 parts
Sodium silicate 80 parts
Potassium sulphate 125 parts
Sodium chloride 139 parts
Sodium carbonate 6,792 parts
Aluminum chloride 1 part
Strontium chloride 1 part
Ammonium chloride 3 parts
Magnesium chloride 24 parts
Calcium chloride 170 parts
Manganese sulphate 0.46 parts
Iron sulphate 1 part
Sulphuric acid 40 parts
Water to make 10 gallons
Mix the first 7 ingredients with about 10 times their weight of water and filter. In the same manner, mix the next 5 ingredients with water and filter; and then the last 3 ingredients. Pour these solutions into sufficient water contained in a fountain to make 10 gallons, and charge at once with carbon dioxide gas. Waters like the above are more correctly named "imitation" than "artificial," as the acidic and basic radicals may bear different relations to one another in the natural and the other.
PURIFYING WATER.
See also Filters.
If an emulsion of clay is poured into a soap solution, the clay gradually separates out without clarifying the liquid. When a few drops of hydrochloric acid, however, are added to a soap solution and a small quantity about 1.5. per cent of a clay emulsion poured in, the liquid clarifies at once, with formation of a plentiful sediment. Exactly the same process takes place when the waste waters from the combing process in spinning are treated with clay. The waters which remain turbid for several days contain 500 to 800 grams of fatty substances per cubic meter. If to 1 liter of this liquid 1 gram of clay is added, with 15 to 20 per cent of water, the liquid clarifies with separation of a sediment and assumes a golden-brown
[741]
WATER - WATERPROOFING
color. Besides the fatty substances, this deposit also contains a certain quantity of nitrogenous bodies. Dried at (100º C.) 212º F., it weighs about 1.6 grams and contains 30 per cent of fat. The grease obtained from it is clear, of good quality, and deliquesces at 95º F. After removal of this fat, the mass still contains 1.19 per cent of nitrogen.
Sterilization of Water with Lime Chloride. In order to disinfect and sterilize 1,000 parts of drinking water, 0.15 parts of dry chloride of lime are sufficient. The lime is stirred with a little water into a thin paste and introduced, with stirring, into the water to be disinfected and a few drops of officinal hydrochloric acid are added. After 1/2 hour the clarification and disinfection is accomplished, whereupon 0.3 parts of calcium sulphite are added, in order to kill the unpleasant smell and taste of the chlorine.
Clarifying Muddy Water. The water supply from rivers is so muddy at times that it will not go through the filter. When this happens agitate each barrel of water with 2 pounds of phosphate of lime and allow it to settle. This will take but a few minutes, and it will be found that most of the impurities have been carried down to the bottom. The water can then be drawn off carefully and filtered.
Removal of Iron from Drinking Water. The simplest method for removing the taste of iron in spring water is to pass the water through a filter containing a layer of tricalcic phosphate either in connection with other filtering materials or alone. The phosphate is first recovered in a gelatinous form, then dried and powdered.
For Hardness. A solution perfectly adapted to this purpose, and one which may be kept a long time, is prepared as follows:
Thirty-five parts of almond oil are mixed with 50 parts of glycerine of
1.26 specific gravity and 8.5 parts of 50 per cent soda lye, and boiled to saponification. To this mixture, when it has cooled to from 85º to 90º C. (185º to 194º F.), are added 100 to 125 parts of boiling water. After cooling again, 500 parts of water are added, and the solution is poured into a quart flask, with 94 per cent alcohol to make up a quart. After standing 2 months it is filtered. Twenty hydrolimeter degrees of this solution make, with 40 parts of a solution of 0.55 grams of barium chloride in 1 quart of water, a dense lather 1 centimeter high.
WATER (COPPER):
See Copper.
WATER ICES:
See Ice Creams.
WATER, TO FREEZE:
See Refrigeration.
WATER JACKETS, ANTI-FREEZING SOLUTIONS FOR:
See Freezing Preventives.
WATER SPOTS, PRIMING FOR:
See Paint.
WATER STAINS:
See Wood.
WATER-LILY ROOTS:
See Pyrotechnics.
WATER, STIRRED YELLOW, SCARLET AND COLORLESS:
See Pyrotechnics.
WATERS (TOILET):
See Cosmetics.
WATER-GLASS CEMENTS:
See Adhesives.
WATER GLASS IN STEREOCHROMATIC PAINTING:
See Stereochromy.
Waterproofing
(See also Enamels, Glazes, Paints, Preservatives, Varnishes.)
Waterproofing Brick Arches. Waterproofing of brick arches is done in the following manner: The masonry is first smoothed over with cement mortar. This is then covered with a special compound on which a layer of Hydrex felt is laid so as to lap at least 12 inches on the transverse seams. Five layers of compound and 5 of felt are used, and special attention is paid to securing tightness around the drain pipes and at the spandrel walls. In fact the belt is carried up the back of the latter and turned into the joint under the coping about 2 inches, where it is held with cement mortar. The waterproofing on the arches is protected with 1 inch of cement mortar and that on the walls with a single course of brickwork.
Waterproofing Bltie Prints. Use refined paraffine, and apply by immersing the print in the melted wax, or more conveniently as follows: Immerse in melted paraffine until saturated, a number of pieces of an absorbent cloth a foot or more square. When withdrawn and cooled they are ready for use at any time.
[742]
WATERPROOFING
To apply to a blue print, spread one of the saturated cloths on a smooth surface, place the dry print on it with a second waxed cloth on top, and iron with a moderately hot flatiron. The paper immediately absorbs paraffine until saturated, and becomes translucent and highly waterproofed. The lines of the print are intensified by the process, and there is no shrinking or distortion. As the wax is withdrawn from the cloths, more can be added by melting small pieces directly under the iron.
By immersing the print in a bath of melted paraffine the process is hastened, but the ironing is necessary to remove the surplus wax from the surface, unless the paper is to be directly exposed to the weather and not to be handled. The irons can be heated in most offices by gas or over a lamp, and a supply of saturated cloths obviates the necessity of the bath. This process, which was originally applied to blue prints to be carried by the engineer corps in wet mines, is equally applicable to any kind of paper, and is convenient for waterproofing typewritten or other notices to be posted up and exposed to the weather.
Waterproof Coatings.
I. Rosin oil, 50 parts; rosin, 30 parts; white soap, 9 parts. Apply hot on the surfaces to be protected.
II. It has been observed that when gluten dried at an ordinary temperature, hence capable of absorbing water, is mixed with glycerine and heated, it becomes water-repelling and suitable for a waterproof paint. One part of gluten is mixed with 1 1/2 parts of glycerine, where
by a slimy mass is obtained which is applied on fabrics subsequently subjected to a heat of 248º F. The heating should not last until all glycerine has evaporated, otherwise the coating becomes brittle and peels off.
Waterproofing Canvas.
I. The canvas is coated with a mixture of the three solutions named below:
1. Gelatin, 50 parts; by weight, boiled in 3,000 parts of water free from lime. 2. Alum, 100 parts, dissolved in 3,000 parts of water. 3. Soda soap dissolved in 2,000 parts of water.
II. Prepare a zinc soap by entirely dissolving 56 parts of soft soap in 125 to 150 parts of water. To the boiling liquid add, with constant stirring, 28 to 33 parts of zinc vitriol (white vitriol). The zinc soap floats on top and forms, after cooling, a hard white mass, which is taken out. In order to clean it of admixed carbonic alkali, it must be remelted in boiling fresh water. Next place 232.5 parts of raw linseed oil (free from mucus) in a kettle with 2.5 parts of best potash, and 5 parts of water. This mass is boiled until it has become white and opaque and forms a liquid, soap-like compound. Now, add sugar of lead, 1.25 parts; litharge, 1 part; red lead, 2 parts; and brown rosin, 10.5 parts. The whole is boiled together about 1 hour, the temperature not being allowed to exceed 212º F., and stirring well from time to time. After this add 15 parts of zinc soap and stir the whole until the metal soap has combined with the oil, the temperature not exceeding 212º F. When the mixture is complete, add a solution of caoutchouc, 1.2 parts, and oil of turpentine, 8.56 parts, which must be well incorporated by stirring. The material is first coated on one side by means of a brush with this composition, which must have a temperature of 158º F. Thereupon hang it up to dry, then apply a second layer of composition possessing the same temperature, which is likewise allowed to dry. The fiber is now filled out, so that the canvas is waterproof.
Waterproofing Corks. For the purpose of making corks as impervious as possible, while at the same time keeping them elastic, saturate them with caoutchouc solution. Dissolve caoutchouc in benzine in the ratio of 1 part of caoutchouc to 19 parts of benzine. Into this liquid lay the corks to be impregnated and subject them to a pressure of 150 to
180 pounds by means of a force pump, so that the liquid can thoroughly enter. The corks thus treated must next be exposed to a strong draught of air until all trace of benzine has entirely evaporated and no more smell is noticeable.
WATERPROOFING FABRICS.
It will be convenient to divide waterproof fabrics into two classes, viz., those which are impervious to water, and those which are water-repellent. It is important to make this distinction, for, although all waterproof material is made for the purpose of resisting water, there is a vast difference between the two classes. The physical difference between them can be briefly summed up as follows: Fabrics which are completely impervious to water comprise oil-skins, mackintoshes, and all materials having a water-resisting film on one or both sides, or in the interior of the fabric. Those coming under the second heading of water-repellent materials do not possess
[743]
WATERPROOFING
this film, but have their fibers so treated as to offer less attraction to the water than the water molecules have for themselves.
The principal members of the first group are the rubber-proofed goods; in these the agent employed is rubber in greater or less quantity, together with other bodies of varying properties. Before enlarging on this class, it will be necessary to give a short description of the chemical and physical properties of rubber.
Rubber, or caoutchouc, is a natural gum exuding from a large number of plants, those of the Euphorbiaceae being the chief source for the commercial variety. The raw material appears on the market in the shape of blocks, cakes, or bottle-shaped masses, according to the manner in which it has been collected. It possesses a dark-brown sometimes nearly black exterior; the interior of the mass is of a lighter shade, and varies from a dingy brown to a dirty white, the color depending on the different brands and sources. In the raw state its properties are very different from what they are after going through the various manufacturing processes, and it has only a few of the characteristics which are generally associated with India rubber. Chemically it is a complex hydrocarbon with the formula C45H36, and appears to consist of a highly porous network of cells having several different rosins in their interstices. It is perfectly soluble in no single solvent, but will yield some of its constituents to many different solvents. At a temperature of 10º C. (50º F.) raw caoutchouc is a solid body and possesses very little elasticity. At 36º C. (97º F.) it is soft and elastic to a high degree, and is capable of being stretched 16 times its length. Further increase of temperature lessens its elastic properties, and at 120º C. (248º F.) it melts. While in the raw condition it has several peculiar properties, one of which is: After stretching, and cooling suddenly while stretched, it retains its new form, and only regains its former shape on being warmed. Another striking feature is its strong adhesive capacity; this property is so powerful that the rubber cannot be cut with a knife unless the blade is wet; and freshly cut portions, if pressed together, will adhere and form a homogeneous mass. From these facts it will be seen how it differs from rubber in the shape of a cycle tire or other manufactured form.
The most valuable property possessed by raw caoutchouc is that of entering into chemical combination with sulphur, after which its elasticity is much increased; it will then bear far greater gradations of heat and cold. This chemical treatment of caoutchouc with sulphur is known as "vulcanizing," and, if properly carried out, will yield either soft vulcanized rubber or the hard variety known as vulcanite. On the other hand, caoutchouc, after vulcanizing, has lost its plastic nature, and can no longer be molded into various shapes, so that in the production of stamped or molded objects, the customary method is to form them in unvulcanized rubber and then to vulcanize them.
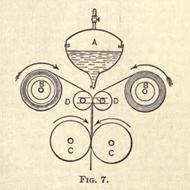
Raw caoutchouc contains a number of natural impurities, such as sand, twigs, soil, etc.; these require removing before the manufacturing processes can be carried out. The first operation, after rough washing, is to shred the raw material into small strips, so as to enable the impurities to DC washed out. This process is carried out by pressing the rubber against the surface of a revolving drum (A, Fig. 1), carrying a
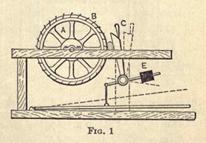
number of diagonally arranged knives, B, on its surface. A lever, C, presses the rubber against the knives; D is the fulcrum on which C works, E being a weight which throws back the lever on the pressure being removed. During
[744]
WATERPROOFING
this operation a jet of water is kept playing onto the knives to cool and enable them to cut.
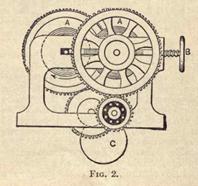
Following this conies the passage between a pair of corrugated steel rollers (as shown in Fig. 2). These rollers have each a different speed, so that the rubber gets stretched and squeezed at the same time. Immediately over the rollers a water pipe is fixed, so that a steady stream of water washes out all the sand and other extraneous matter. In Fig. 2, A A are the steel rollers, while B is a screw working springs which regulate the pressure between the rollers. The power is transmitted from below from the pulley, C, and thence to the gearing.
The next operation, after well drying, is to thoroughly masticate the shredded rubber between hot steel rollers, which resemble those already described, but usually have a screw-thread cut on their surfaces. Fig. 3 shows the front view
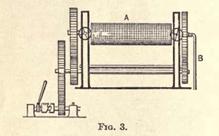
of this masticating machine, A being the rollers, while the steam pipe for heating is shown at B. Fig. 3a gives a top view
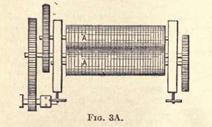
of the same machine, showing the two rollers.
After passing several times through these, the rubber will be in the form of homogeneous strips, and is then ready either for molding or dissolving. As we are dealing solely with waterproofed textiles, the next process which concerns us is the dissolving of the rubber in a
suitable solvent. Benzol, carbon bisulphide, oil of turpentine, ether, and absolute alcohol, will each dissolve a certain amount of rubber, but no one of them used alone gives a thorough solution. The agent commonly employed is carbon bisulphide, together with 10 per cent of absolute alcohol. Whatever solvent is used, after being steeped in it
for some hours the caoutchouc swells out enormously, and then requires the addition of some other solvent to effect a complete solution. A general method is to place the finely shredded rubber in a closed vessel, to cover it with carbon bisulphide, and allow to stand for some
hours. Toward the end of the time the vessel is warmed by means of a steam coil or jacket, and 10 parts absolute alcohol are added for every 100 parts of carbon bisulphide. The whole is then kept gently stirred for a few hours. Fig. 4 shows a common type of the vessel
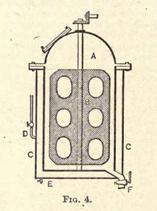
used for dissolving rubber. In this diagram A is the interior of the vessel, and B a revolving mixer in the same. The whole vessel is surrounded by a steam jacket, C, with a steam inlet at D and a tap for condensed water at E. F is the cock by which the solution is drawn off.
After the rubber is dissolved, about 12 to 24 per cent of sulphur is added, and thoroughly incorporated with the solution. The sulphur may be in the form of chloride of sulphur, or as sulphur pure and simple. A very small quantity of sulphur is required to give the necessary result, 2 to 3 per cent being sufficient to effect vulcanization; but a large quantity is always added to hasten the operation.
Even after prolonged treatment with the two solvents, a solution of uniform consistency is never obtained: clots of a thicker nature will be found floating in the solution, and the next operation is to knead it up so as to obtain equal
[745]
WATERPROOFING
density throughout. Fig. 5 will give an idea of how this mixing is done.
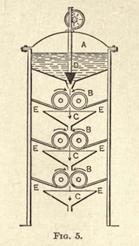
At the top of a closed wooden chamber is a covered reservoir, A, containing the solution of rubber. A long slit at the base of this reservoir allows the solution to fall between sets of metal rollers, BBB below. Neighboring rollers are revolving in opposite directions, and at different speeds, so that, after passing all three sets of rollers, and emerging at the bottom, the solution should be of uniform consistency. CCC are the guiding funnels, and EE are scrapers to clear the solution from the rollers. D is a wedgeshaped plug worked by a rack and pinion, and regulates the flow of the solution.
It now remains to apply the rubber to the fabric and vulcanize it. Up to this stage the sulphur has only been mechanically mixed with the rubber; the aid of heat is now required to bring about chemical combination between the two. This process, which is known as "burning," consists in subjecting the rubber covered fabric to a temperature of about 248º F. Sulphur itself melts at 239º F.,
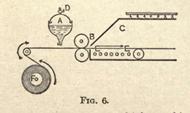
and the temperature at which combination takes place must be above this. Fig. 6 shows one of the methods of spreading the rubber on the cloth. A is the tank containing the solution with an outlet at the bottom arranged so as to regulate the flow of solution. The fabric passes slowly underneath this, receiving as it travels a thin coating of the waterproofing. The two rollers at B press the solution into the fabric and distribute the proofing evenly over the entire surface.
After leaving the two squeezing rollers, the cloth travels slowly through a covered chamber, C, having a series of steam pipes, EE, underneath, to evaporate the solvent; this condenses on the upper portion of the chamber, which is kept cooled, and flows down the sides into suitable receptacles. After this the proofed cloth is vulcanized by passing round metal cylinders heated to the necessary temperature, or by passing through a heated chamber. Fig. 7 shows the spreading of
rubber between two fabrics. The two cloths are wound evenly on the rollers, BB; from this they are drawn conjointly through the rollers, D, the stream of proofing solution flowing down between the rollers, which then press the two fabrics together with the rubber inside.
The lower rollers marked CC are heated to the necessary degree, and cause the rubber and sulphur to combine in chemical union.
So far the operation of proofing has been described as though pure rubber only was used; in practice the rubber forms only a small percentage of the proofing material, its place being taken by cheaper bodies. One of the common ingredients of proofing mixtures is boiled linseed oil together with a small quantity of litharge; this dries very quickly, and forms a glassy flexible film. Coal tar, shellac, colophony, etc., are all used, together with India-rubber varnish, to make
[746]
WATERPROOFING
different waterproof compositions. Oil of turpentine and benzol form good solvents for rubber, but it is absolutely essential that both rubber and solvent be perfectly anhydrous before mixing. Oil of turpen-
tine, alcohol, etc., can be best deprived of water by mixing with either sulphuric acid or dehydrated copper sulphate, and allowing to stand. The acid or the copper salt will absorb the water and sink to the bottom, leaving a supernatant layer of dehydrated turpentine "or whatever solvent is used. All the sulphur in a rubber-proofed cloth is not in combination with the rubber; it is frequently found that, after a lapse of time, rubber-proofed material shows an efflorescence of sulphur on the surface, due to excess of sulphur, and occasionally the fabric becomes stiff and the proofing scales off. Whenever a large proportion of sulphur is present, there is always the danger of the rubbers forming slowly into the hard vulcanite state, as the substance commonly called vulcanite consists only of ordinary vulcanized rubber carried a stage further by more sulphur being used and extra heat applied. If after vulcanizing, rubber is treated with caustic soda, all this superfluous sulphur can be extracted; if it is then well washed the rubber will retain its elasticity for a long period. With the old methods of proofing, a sheet of vulcanized rubber was cemented to a fabric with rubber varnish, and frequently this desulphurizing was performed before cementing together. The result was a flexible and durable cloth, but of great weight and thickness, and expensive to produce.
The chemistry of rubber is very little understood; as mentioned previously, rubber is a highly complex body, liable to go through many changes. These changes are likely to be greater in rubber varnish, consisting of half a dozen or more ingredients, than in the case of rubber alone. The action of sunlight has a powerful effect on rubber, much to its detriment, and appears to increase its tendency to oxidize. Vulcanized rubber keeps its properties better under water than when exposed to the air, and changes more slowly if kept away from the light. It appears as though a slight decomposition always takes place even with pure rubber; but the presence of so many differently constituted substances as sometimes occur in rubber solutions no doubt makes things worse. Whenever a number of different bodies with varying properties are consolidated together by heat, as in the case of rubber compositions, it is only reasonable to expect there will be some molecular rearrangement going on in the mass; and this can be assigned as the reason why some proofings last as long again as others. Some metallic salts have a very injurious action on rubber, one of the worst being copper sulphate. Dyers are frequently warned that goods for rubber-proofing must be free from this metal, as its action on rubber is very powerful, though but little understood. As is generally known, grease in any form is exceedingly destructive to rubber, and it should never be allowed in contact in the smallest proportion. Some compositions are made up by dissolving rubber in turpentine and coal tar; but in this case some of the rubber's most valuable properties are destroyed, and it is doubtful if it can be properly vulcanized.
Owing to rubber being a bad conductor of heat, it requires considerable care to vulcanize it in any thickness. A high degree of heat applied during a short period would tend to form a layer of hard vulcanite on the surface, while that immediately below would be softer and would gradually merge into raw rubber in the center.
The different brands of rubber vary so much, especially with regard to solubility, that it is always advisable to treat each brand by itself, and not to make a solution of two or more kinds. Oilskins and tarpaulins, etc., are mostly proofed by boiled linseed oil, with or without thickening bodies added. They are not of sufficient interest to enlarge upon in this article, so the second, or "water-repellent," class has now to be dealt with.
All the shower-proof fabrics come under this heading, as well as every cloth which is pervious to air and repulsive to water. The most time-honored recipe for proofing woollen goods is a mixture of sugar of lead and alum, and dates back hundreds of years. The system of using this is as follows: The two ingredients are dissolved separately, and the solutions mixed together. A mutual decomposition results, the base of the lead salt uniting with the sulphuric acid out of the alum to form lead sulphate, which precipitates to the bottom. The clear solution contains alumina in the form of acetate, and this supplies the proofing quality to the fabric. It is applied in a form of machine shown in Fig. 8, which will be seen to consist of a trough containing the proofing solution, (7, with a pair of squeezing rollers, A, over the top.
The fabric is drawn down through the solution and up through the squeezers in the direction of the arrows. At the
[747]
WATERPROOFING
back of the machine the cloth automatically winds itself onto a roll, J9, and then only requires drying to develop the water
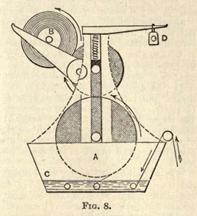
resisting power. D is a weight acting on a lever which presses the two rollers, A, together. The water-repelling property is gained as follows:
Drying the fabric, which is impregnated with acetate of alumina, drives off some of the volatile acetic acid, leaving a film of basic acetate of alumina on each wool fiber. This basic salt is very difficult to wet, and has so little attraction for moisture that in a shower of rain the drops remain in a spheroidal state, and fall off. In a strong wind, or under pressure, water eventually penetrates through fabrics proofed in this manner; but they will effectually resist a sharp shower.
Unfortunately, shower-proofed goods, with wear, gradually lose this property of repelling water. The equation representing the change between alum and sugar of lead is given below. In the case of common alum there would, of course, be potassium acetate in solution besides the alumina.
Alum Sugar Lead Potassium Aluminum
of lead sulphate acetate acetate
Al2K2(S04)4 + 4Pb(C2H3O2)2 = 4PbSO4 + 2KC2H3O2 + A12(C2H3O2)8
Now that sulphate of alumina is in common use, alum need not be used, as the potash in it serves no purpose in proofing.
There are many compositions conferring water: resisting powers upon textiles, but unfortunately they either affect the general handle of the material and make it stiff, or they stain and discolor it, which is equally bad. A large range of waterproof compositions can be got by using stearates of the metals; these, in nearly every case, are insoluble bodies, and when deposited in the interior of a fabric form a water-resisting "filling" which is very effective. As a rule these stearates are deposited on the material by means of double baths; for example, by passing the fabric through (say) a bath of aluminum acetate, and then, after squeezing out the excess of liquid, passing it through a bath of soap. The aluminum salt on the fabric decomposes the soap, resulting in a deposit of insoluble stearate of alumina. This system of proofing in two baths is cleaner and more economical than adding all the ingredients together, as the stearate formed is just where it is required "on the fibers," and not at the bottom of the bath.
One of the most important patents now worked for waterproofing purposes
is on the lines of the old alumina process. In this case the factor used is rosin, dissolved in a very large bulk of petroleum spirit. The fabrics to be proofed (usually dress materials) are passed through a bath of this solution, and carefully dried to drive off the solvent.
Following this, the goods are treated by pressing with hot polished metal rollers. This last process melts the small quantity of rosin, which is deposited on the cloth, and leaves each single fiber with an exceedingly thin film of rosin on it. It will be understood that only a very attenuated solution of rosin is permissible, so that the fibers of the threads and not the threads themselves are coated with it. If the solution contains too much rosin the fabric is stiffened, and the threads cemented together; whereas if used at the correct strength (or, rather, weakness) neither fabric nor dye suffers, and there is no evidence of stickiness of any description.
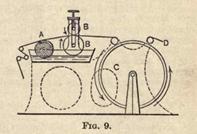
Fig. 9 shows a machine used for spreading a coat of either proofing or any other fluid on one side of the fabric.
[748]
WATERPROOFING
This is done by means of a roller, A, running in the proofing solution, the material to be coated traveling slowly over the top and just in contact with the roller, A, which transfers the proofing to it. Should the solution used be of a thick nature, then a smooth metal roller will transfer sufficient to the fabric. If the reverse is the case, and the liquid used is very thin, then the roller is covered with felt, which very materially adds to its carrying power. As shown in Fig. 9, after leaving the two squeezing rollers, BB, the fabric passes slowly round a large steam-heated cylinder, C, with the coated side uppermost. This dries the proofing and fastens it, and the cloth is taken off at D.
Besides stearates of the metals, glues and gelatins have been used for proofing purposes, but owing to their stiffening effect, they are only of use in some few isolated cases. With glue and gelatin the fixing agent is either tannic acid or some metallic salt. Tannic acid converts gelatin into an insoluble leather-like body; this can be deposited in the interstices of the fabric by passing the latter through a gelatin bath first, and then squeezing and passing through the tannic acid. Bichromate of potash also possesses the property of fixing the proteid bodies and rendering them insoluble.
The following are special processes used to advantage in the manufacture of waterproof fabrics:
I. Ordinary Fabrics, Dressing Apparel, etc. Immerse in a vat of acetate of alumina (5º Bé.) for 12 hours, lift, dry, and let evaporate at a temperature of from 140º to 149º F.
II. Sailcloth, Awnings, Thick Blankets, etc. Soak in a 7 per cent solution of gelatin at 104º F., dry, pass through a 4 per cent solution of alum, dry again, rinse in water, and dry.
III. Fabrics of Cotton, Linen, Jute, and Hemp. Put into a bath of ammoniacal cupric sulphate of 10º Bé. at a temperature of 87º F.; let steep thoroughly, then put in a bath of caustic soda (20º Bé.) and dry. To increase the impermeability, a bath of sulphate of alumina may be substituted for the causticsoda bath.
IV. Saturate the fabrics with the following odorless compound, subjecting them several times to a brushing machine having several rollers, where the warp threads will be well smoothed, and a waterproof product of fine sheen and scarcely fading will be the result. The compound is made with 30 parts, by weight, of Japan wax, 22 1/2 parts, by weight, of paraffine, 12 parts, by weight, of rosin soap, 35 parts, by weight, of starch, and 5 parts, by weight, of a 5 per cent solution of alum. Fabrics thus prepared are particularly adapted to the manufacture of haversacks, shoes, etc.
V. White or Light Fabrics. Pass first through a bath of acetate of alumina of 4º to 5º Bé. at a temperature of 104º F., then through the rollers to rid of all liquid; put into a warm solution of soap
(5 parts, by weight, of olive-oil soap to 100 parts, by weight, of fresh water) and finally pass through a 2 per cent solution of alum, dry for 2 or 3 days on the dropping horse, and brush off all particles of soap.
VI. Dissolve 1 1/2 parts, by weight, of gelatin in 50 parts, by weight, of boiling water, add 1 1/2 parts, by weight, of scraped tallow soap and 2 1/2 parts, by weight, of alum, the latter being put in gradually; lower the temperature of the bath to 122º F., lift out the fabric, dry, and calender.
VII. Tent Cloth. Soak in a warm solution of 1 part, by weight, of gelatin, 1 part, by weight, of glycerine, and 1 part, by weight, of tannin in 12 parts, y weight, of wood vinegar (pyroligneous acid) of 12º Bé. The whole is melted in a kettle and carefully mixed. The mass is poured into the receiver of the brushing machine, care being taken to keep it liquid. For a piece of 500 feet in length and 20 inches in width, 50 to 80 parts, by weight, of this compound are needed.
VIII. To freshen worn waterproof material, cover with the following: Fifty-five thousand parts, by weight, of gelatin; 100 parts, by weight, of bichromate of potash; 100 parts, by weight, of acetic acid (to keep glue from congealing), and from 3,000 to 5,000 parts, by weight, of water; to this add 500 parts, by weight, of peroxide of ammoniacal copper, 100º Bé. This compound is put on the fabric with a brush and then exposed to air and light.
IX. Soft Hats. The hats are stiffened as usual, then put through the following three baths: Dissolve 1/2 part, by weight, of tallow soap in from 40 to 50 parts, by weight, of warm water (140º F.). Put 3 to 4 dozen hats into this solution, leave them in it for half an hour, then take out and put them as they are into another bath prepared with 40 to 50 parts, by weight, of water and 1/2 part, by weight, of alum and heated to 86 to 104º F. After
[749]
WATERPROOFING
having been left in the second bath for 1/4 or 1/2 hour, take out as before, put into the third bath of 40 to 50 parts, by weight, of water, 1/2 part, by weight, of alum, and about 13 parts, by weight, of fish glue. In this cold bath the hats are left for another 1/2 hour or more until they are completely saturated with the liquid, then dried and the other operations continued.
X. Woolen cloth may be soaked in a vat filled with aluminum acetate, of 5º Bé., for 12 hours, then removed, dried, and dried again at a temperature of 140º F.
XI. Wagon covers, awnings, and sails are saturated with a 7 per cent gelatin solution, at a temperature of 104º F., dried in the air, put through a 4 per cent solution of alum, dried again in the air, carried through water, and dried a third time.
XII. Cotton, linen, jute, and hemp fabrics are first thoroughly saturated in a bath of ammonio-cupric sulphate, of 10º Bé., at a temperature of 77º F., then put into a solution of caustic soda, 2
Be., and dried. They may be made still more impervious to water by substituting a solution of aluminum sulphate for the caustic soda.
XIII. White and light-colored fabrics are first put into a bath of aluminum acetate, 4º to 5º Bé., at a temperature of 102º F., the superfluous liquid being removed from the fabric by press rollers. The fabric is put into a soap solution (5 parts of good Marseilles soap in 100 parts of soft water). Finally it is put through a 2 per cent alum solution, and left to dry for 2 or 3 days on racks. The adhering particles of soap are removed by brushing with machinery.
XIV. Dissolve 1.5 parts of gelatin in 50 parts of boiling water, add 1 .5 parts of shavings of tallow grain soap, and gradually, 2.5 parts of alum. Let this cool to 122º F., draw the fabric through it, dry and calender.
XV. Cellular tissues are made waterproof by impregnating them with a warm solution of 1 part, by weight, of gelatin, 1 part, by weight, of glycerine, and 1 part, by weight, of tannin, in 12 parts, by weight, of wood vinegar, 12º Bé.
XVI. Linen, hemp, jute, cotton, and other fabrics can be given a good odorless waterproof finish by impregnating them, and afterwards subjecting them to the action of several mechanical brush rollers. By this process the fabric is brushed dry, the fibers are laid smooth, the threads of the warp brought out, and a glossy, odorless, unfading waterproof stuff results. Fabrics manufactured in the usual way from rough and colored yarns are put through a bath of this waterproof finish, whose composition is as follows: Thirty parts, by weight, of Japanese wax; 22.5 parts, by weight, of paraffine; 15 parts, by weight, of rosin soap; 35 parts, by weight, of starch, and 5 parts, by weight, of a 5 per cent alum solution. The first three components are melted in a kettle, the starch and, lastly, the alum added, and the whole stirred vigorously.
XVII. One hundred parts, by weight, of castor oil are heated to nearly 204º F., with 50 parts, by weight, of caustic potash, of 50 Be, to which 50 parts, by weight, of water have previously been added. Forty parts, by weight, of cooler water are then added slowly, care being taken to keep the temperature of the mixture constant. As soon as the liquor begins to rise, 40 parts, by weight, of cooler water are again added, with the same precaution to keep the temperature from falling below 204º F. At the same time care must be taken to prevent the liquor boiling, as this would produce too great saponification. By the prolonged action of heat below the boiling point, the oil absorbs water and caustic potash without being changed, and the whole finally forms a perfectly limpid, nearly black liquid. This is diluted with 5 times its weight of hot or cold water, and is then ready for use without any further preparation. Other vegetable oils may be employed besides castor oil, and the quantity of unsaponified oil present may be increased by stirring the prepared liquid with a fresh quantity of castor or other vegetable oil. The product is slightly alkaline, but wool fiber is not injured, as the oiling may be done in the cold. The solution is clear and limpid, and will not separate out on standing like an emulsion. This product in spinning gives a 10 per cent better utilization of the raw material owing to the greater evenness and regularity with which the fibers are oiled; in weaving less oiling is required.
The product can be completely removed by water, preferably by cold water, and scouring of the goods subsequently with soap, soda, or fuller's earth can thus be dispensed with.
XVIII. Cloth may be rendered waterproof by rubbing the under side with a lump of beeswax until the surface presents a uniform white or grayish appearance. This method it is said renders the cloth
[750]
WATERPROOFING
practically waterproof, although still leaving it porous to air.
XIX. Coating the under side of the cloth with a solution of isinglass and then applying an infusion of galls is another method, a compound being thus formed which is a variety of leather.
XX. An easy method is the formation of aluminum stearate in the fiber of the cloth, which may readily be done by immersing it in a solution of aluminum sulphate in water (1 in 10) and without allowing it to dry passing through a solution of soap made from soda and tallow or similar fat, in hot water. Reaction between the aluminum sulphate and the soap produces aluminum stearate and sodium sulphate. The former is insoluble and remains in the fiber; the latter is removed by subsequently rinsing the fabric in water.
XXI. A favorite method for cloth is as follows: Dissolve in a receptacle, preferably of copper, over a bright coal fire, 1 liter (1.76 pints) of pure linseed oil, 1 liter (1.76 pints) of petroleum, 1/2 liter (0.88 pints) of oil turpentine, and 125 grams (4.37 ounces) of yellow wax, the last named in small bits. As there is danger of fire, boiling of this mass should be avoided. With this hot solution removed from the fire, of course the felt material is impregnated; next it is hung up in a warm, dry room or spread out, but in such a manner that the uniform temperature can act upon all parts.
Waterproofing Leather.
I. Tenning's process is as follows: Melt together equal parts of zinc and linseed oil, at a temperature not above 225º F. Put the leather in the molten mixture and let it remain until saturated. The "zinc soap" is made by dissolving 6 parts of white soap in 16 parts of water, and stirring into the solution 6 parts of zinc sulphate. To make sure of a homogeneous mixture remelt the whole and stir until it begins to cool. The process, including the saturation of the leather, requires about 48
hours. Instead of zinc sulphate, copper or iron sulphate may be used. the philosophy of the process is that the moisture and air contained in the pores of the leather are driven out by the heat of the soap mixture, and their place is taken, on cooling, by the mixture. The surface of the leather is scraped after cooling, and the article is dried, either by heating over an open fire or by hanging in a drying room, strongly heated.
II. Prideaux' process consists in submitting the leather to treatment with a solution of caoutchouc until it is thoroughly saturated with the liquid. The latter consists of 30 parts of caoutchouc in 500 parts of oil of turpentine. Complete impregnation of the leather requires several days, during which the solution must be frequently applied to the surface of the leather and rubbed in.
III. Villon's process consists in applying a soap solution to the leather, about as follows: The leather is first treated to a solution of 62 parts of soap, 124 parts of glue, and 2,000 parts of water. When it has become saturated with the solution, it is treated to rubbing with a mixture of 460 parts of common salt and 400 parts of alum, in sufficient water to dissolve the same. After this it is washed with tepid water and dried. This process is much the quickest. The application of the soap requires about 2 hours, and the subsequent treatment about as much more, or 4 or 5 hours in all.
Oilskins. The art of painting over textile fabrics with oily preparations to make them waterproof is probably nearly as old as textile manufacture itself, an industry of prehistoric, nay, geologic, origin. It is certainly more ancient than the craft of the artistic painter in oils, whose canvases are nothing more nor less than art oilskins, and when out of their frames, have served the usual purpose of those things in protecting goods or the human body before now. The art of waterproofing has been extended beyond the domain of the oilskin by chemical processes, especially those in which alum or lead salts, or tannin, are used, as well as by the discovery of India rubber and
gutta percha. These two have revolutionized the waterproofing industry in quite a special manner, and the oilskin manufacture, although it still exists and is in a fairly flourishing condition, has found its products to a very large extent replaced by rubber goods. The natural result has been that the processes used in the former industry have remained now unchanged for a good many years. They had already been brought to a very perfect state when the rubber-waterproofing business sprang up, so that improvements were even then difficult to hit upon
in oilskin making, and the check put upon the trade by India rubber made people less willing to spend time and money in experimenting with a view to improving what many years had already made it difficult to better. Hence the three cardinal defects of the oilskin: its weight, its stiffness, and the liability of