The
Science Notebook
Lionel Chem-Lab
- Chapter 5
The
Science Notebook
Lionel Chem-Lab
- Chapter 5
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 79 - 101
CHAPTER V
THE STORY OF CARBON AND ITS COMPOUNDS
Carbon is familiar to everyone because of its
widespread occurrence in nature. There are so many compounds
composed of carbon in combination with hydrogen, oxygen and other
elements that the branch of chemistry which deals with them has a
special name, organic chemistry.
In its free state, carbon is found as diamond, graphite and coal. The purest carbon is
the beautiful precious gem known as a diamond. Graphite and coal
contain more or less free carbon but in an entirely different
form.
Every living thing, plant or animal, has carbon
in its tissues in the form of organic compounds. Carbon dioxide,
which we exhale from our lungs, is the most familiar gaseous
compound of carbon. Natural gas and petroleum are other compounds
of carbon and hydrogen.
This element also occurs abundantly in the carbonates of certain metals,
especially calcium and magnesium. Marble and limestone are two
varieties of calcium carbonate. A considerable part of the earth’s
crust consists of these materials.
Our bodies and the food we eat also contain
large percentages of the compounds of carbon and this is such an
important phase of chemistry that we shall later devote a whole
chapter of our book to it.
In the free state, carbon occurs in both the
crystalline and the amorphous, or non-crystalline, form. Diamonds
and graphite are crystalline forms of carbon. Although very
brittle, the diamond is one of the hardest substances known to
man. In addition to its value as a thing of beauty, it has many
uses in industry where an extremely hard cutting tool is required.
Graphite, the second form of crystalline
carbon, is a shiny black substance, very soft and slippery to the
touch, used in the manufacture of lead pencils and crucibles, as a
lubricant, and in the form of a polish or paint as a protective
covering for iron.
PROPERTIES OF CARBON
Pure amorphous (non-crystalline) carbon is best prepared by heating
sugar in the absence of air. Hydrogen and oxygen are expelled
(largely in
79
80 THE
STORY OF CARBON
the form of water) and pure carbon remains. Almost any form of
organic matter yields carbon when similarly heated.
While the various forms of carbon differ in many respects,
especially in hardness, yet they are all odorless, tasteless solids,
insoluble in water.
BONEBLACK
Boneblack or animal charcoal can be obtained by
heating bones in the absence of air. The product consists of
calcium phosphate and very finely divided carbon. Boneblack
is used extensively for filter purposes. It has the ability to
absorb both coloring material and gases from solution and is
especially valuable in removing coloring material in the refining
of sugar. The name given to this property is adsorption (not to be
confused with absorption).
EXPERIMENT No. 136 Bitter Taste Of Quinine
Removed by Charcoal
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Test tube, quinine (drug store) and charcoal.
PROCEDURE:
Add a few grains of quinine to a test tube one quarter full of
water. Shake well and put a drop of the solution on the
tongue. Place three measures of charcoal in the test tube and
again shake well. Filter off the charcoal and taste a drop of
the solution. Continue adding charcoal and subsequent filtering
until the bitter taste is no longer present.
SUMMARY:
Due to the adsorptive properties of charcoal, the bitter taste of
quinine is removed from the solution.
GAS MASKS
Carbon is an extremely valuable defense
material because of its property of adsorbing large volumes of
gases. This makes it an ideal material to use in the manufacture
of gas masks. Charcoal prepared especially for adsorbing gases is
known as activated carbon.
The volume of gas adsorbed by any given carbon depends upon how it
was made. In the manufacture of gas masks, carbon prepared from
cocoanut shells and peach stones is one of the best. Gas masks
developed during the first World War to protect troops from
poisonous gas were used after the war to protect coal miners and
other workmen.
EXPERIMENT No. 137 Deodorizing Properties Of
Charcoal
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered charcoal, paraffin, sulfur, delivery tube and stopper,
funnel, filter paper, candle or alcohol lamp, two test tubes.
PROCEDURE:
Place a small piece of paraffin and three measures of
LIONEL
CHEM-LAB 81
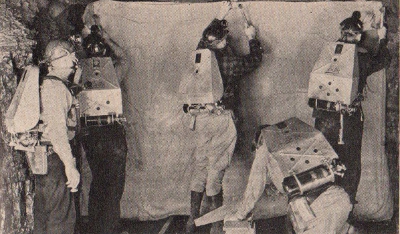
U.S. Bureau of Mines
An emergency mine crew wearing gas masks which supply
each man with oxygen. These men are able to work in a
gas-filled mine in perfect safety.
sulfur in a test tube. Insert the delivery tube and stopper with the
long stem running into a test tube half full of water. Heat test
tube and contents noting the odor of the hydrogen sulfide. Add four
measures of powdered charcoal to the hydrogen sulfide water and
shake the tube vigorously. Filter the solution and collect the
filtrate in another test tube. Note that the odor has practically
disappeared.
SUMMARY:
When liquids having a disagreeable odor are mixed with activated
carbon and then filtered, the gaseous impurities responsible for the
odor are extracted. What happens is that the molecules of the gases
collect on the surface of the porous charcoal and stay behind with
the charcoal when it is filtered out of the liquid.
COAL
Coal is formed from vast accumulations of
vegetable matter. Millions of years ago, the earth was covered
with massive forests and jungles of giant ferns. As these great
forests toppled into swamps, the decaying vegetation was crushed
deep into the soil and transformed by the pressure of centuries
into carbon, diamonds and coal.
There are two chief forms of coal, anthracite and bituminous. Neither one in
pure carbon. In hard coal (anthracite) nearly all the carbon is
uncombined, while in soft coal (bituminous) a considerable portion
of the carbon is in combination with hydrogen, oxygen, nitrogen
and sulfur.
82 THE
STORY OF CARBON
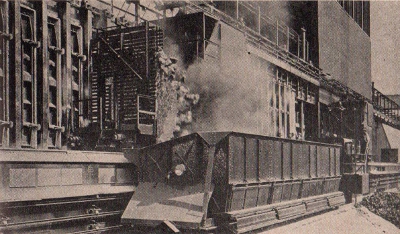
Coal dust is ignited in
experiments conducted by the U. S. Bureau of Mines to
determine the causes of coal mine explosions.
When soft coal is heated in the absence of
air, certain changes take place which result in the formation of a
number of very useful carbon compounds. These compounds are
released in the form of gases and vapors, the mineral matter and
free carbon remaining as a solid. This process is known as destructive distillation and
the matter which escapes in this process is known as volatile matter. The solid
that remains is coke. In soft coal, the percentage of volatile
matter is much greater than in hard coal.
EXPERIMENT No. 138 Distillation Of Coal
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered soft coal, test tube, candle or alcohol lamp, glass rod,
delivery tube and stopper.
PROCEDURE:
Place four measures of powdered coal in a test tube and heat. Hold a
cold glass rod at the mouth of the test tube. Note the substance
which adheres to the glass rod. Attach delivery tube and stopper to
test tube and continue heating. Ignite the gas which flows from the
gas delivery tube (discontinue heating when smoking in the tube
stops). Examine the substance remaining in test tube.
SUMMARY:
When soft coal undergoes destructive distillation, the most
important solid by-product is coke which is used as a fuel and a
reducing agent. The substance adhering to the glass rod is another
by-product called pitch, a tarry mixture used in road construction.
The combustible gas liberated in coal gas consisting chiefly of
hydrogen, methane and a little carbon monoxide.
LIONEL
CHEM-LAB 83
COKE AND COAL GAS
It has been known for a good many years that
the heating of soft coal in the absence of air releases certain
combustible gases. In fact such gas was used for street lighting
for over a hundred years. Other very valuable byproducts are
ammonia and a thick, gummy substance known as coal tar which can
be broken down chemically into thousands of useful compounds -
dyes, medicines, disinfectants and explosives.
General Electric Co.
A by-product coke oven of the Great Lakes Steel
Corporation.
In the manufacture of coal gas, therefore, not only the gas itself
is obtained but also coke, ammonia and coal tar. Coke is a very
important product not only as a fuel but also as a reducing agent in
separating materials, like iron, from their ores.
The quantity of coke obtained in the
manufacture of coal gas has never been sufficient to meet the
demand and formerly there was a great deal of waste in producing
the additional coke since all the by-products escaped into the
air. This wasteful process has now given way almost entirely
to the use of the by-product
coke oven, which makes it possible to save all the
products formed.
Coke plus lime in the electric furnace gives
calcium carbide, which upon the addition of water, produces acetylene gas.
Acetylene, once used in automobile and bicycle headlamps, and
still used in the oxy-acetylene blow torch
84 THE STORY OF CARBON
for cutting and welding metals, has of late
filled a more important role as a chemical intermediate. Acetylene
gas plus hydrochloric acid are the raw materials from which neoprene - chemical rubber -
is produced.
HOW CHARCOAL IS MADE
Charcoal is prepared from wood just as coke is
prepared from coal and the volatile matter which is given off by
the destructive distillation of wood likewise contains many
valuable substances.
EXPERIMENT N0. 139 Distillation Of Wood In
Absence Of Air
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Five wooden matches, test tube, test tube holder, candle or alcohol
lamp, blue litmus paper, delivery tube and stopper.
PROCEDURE:
Break off the match heads and place the sticks in the test tube.
Heat the tube until the wood begins to smoke. Expose a strip of
moistened blue litmus paper at the mouth of the test tube. Note that
it turns red. Attach stopper and delivery tube and continue heating.
Light the gas coming out of the delivery tube and continue the
heating as long as gas forms. Allow to cool. Remove stopper and
notice the charcoal remaining in the test tube.
SUMMARY:
Some of the products of the destructive distillation of wood are
charcoal, acetic acid, methyl or wood alcohol, road tar and acetone.
The gas is combustible and can be used for heating.
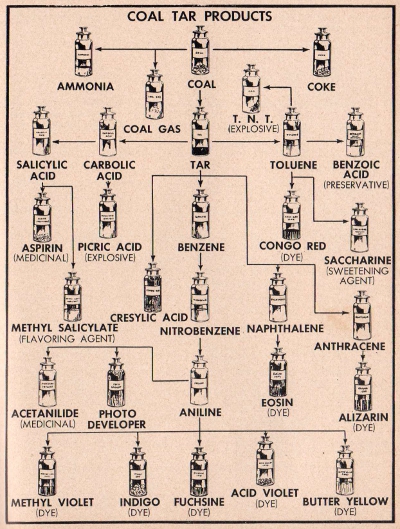
COMPOSITION OF COAL TAR
Coal tar is a black substance composed of a
variety of compounds, the six most important being benzine, toluene, carbolic acid (phenol), cresylic acid, naphtholene and anthracene. Each one of these
materials in turn is used in the preparation of thousands of other
compounds. The chart on page 85 shows the variety of materials
derived from coal tar. For example, toluene is used in the
manufacture of a very powerful explosive known as TNT, and it is
also used in the preparation of such diverse products as saccharin
and congo red. Cresylic acid is used to make disinfectants.
Carbolic acid is a very important industrial chemical,
indispensable in the production of modern plastic materials.
Benzine is used to prepare nitro-benzine which in turn is used to
manufacture aniline. All dyes derived from coal tar are known as
aniline dyes.
PHENOL
Phenol, commonly known as carbolic acid, is a
coal tar derivative whose principal uses are in the making of
plastic materials, picric acid for explo-
LIONEL
CHEM-LAB 85
Diagram
of the variety of important products obtainable from common coal
tar.
86 THE
STORY OF CARBON
sives and making disinfectants. One of our
experiments is to make phenol from sodium salicylate.
EXPERIMENT No. 140 Phenol From Sodium Salicylate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium salicylate, calcium oxide, alcohol lamp or candle, test tube.
PROCEDURE:
Mix two measures of sodium salicylate and an equal amount of calcium
oxide in a test tube. Heat carefully until fumes begin to come
off. Discontinue heating and cautiously smell the odor present.
SUMMARY:
If the fumes smell like carborated vaseline you have made phenol.
EXPERIMENT No. 141 Forming Salicylic Acid
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium salicylate, sodium bisulfate, test tubes, alcohol lamp or
candle.
PROCEDURE:
Dissolve one measure of sodium bisulfate in a test tube half filled
with water. Dissolve one measure of sodium salicylate in another
test tube half filled with water. Mix with the sodium bisulfate
solution and note the precipitate which gradually appears. Heat
carefully and note that the precipitate dissolves.
SUMMARY:
This precipitate is salicylic acid which is insoluble in cold water
but soluble in hot water.
EXPERIMENT No. 142 How To Make Chromium
Salicylate
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Chrome alum, sodium salicylate and two test tubes.
PROCEDURE:
Dissolve three measures of sodium salicylate in a test tube half
full of water. Dissolve two measures of chrome alum in another test
tube one fourth full of water. Pour into this a few drops of the
sodium salicylate solution and note the red color.
SUMMARY:
The red substance is chromium salicylate.
EXPERIMENT No. 143 How To Make Ferric Salicylate
(CLr-33, CL-44,I,CL-55, CL-66, CL-77)
APPARATUS:
Sodium salicylate, ferric ammonium sulfate, two test tubes.
PROCEDURE:
Dissolve three measures of sodium salicylate in a test tube half
full of water. Dissolve one measure of ferrous ammonium sulfate in
another test tube half full of water. Add to this a few drops of
sodium salicylate solution and note the deep purple color.
SUMMARY:
The deep purple substance is ferric salicylate.
LIONEL
CHEM-LAB 87
EXPERIMENT No. 144 How To Make Ferrous Salicylate
(CL-55, CL-66, CL-77)
APPARATUS:
Ferrous ammonium sulfate, sodium salicylate and two test tubes.
PROCEDURE:
Dissolve three measures of sodium salicylate in a test tube half
full of water. Dissolve one measure of ferrous ammonium sulfate in
another test tube one quarter full of water. Pour into this a few
drops of sodium salicylate solution and note the reddish-brown
precipitate.
SUMMARY:
The reddish-brown precipitate is ferrous salicylate.
EXPERIMENT No. 145 How To Make Copper Salicylate
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, sodium salicylate and two test tubes.
PROCEDURE:
Dissolve three measures of sodium salicylate in a test tube half
full of water. Dissolve one measure of copper sulfate in another
test tube one fourth full of water. Pour into this a few drops
of the sodium salicylate solution and note the green color.
SUMMARY:
The green substance is copper salicylate.
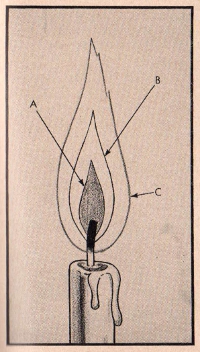
FIGURE 14
CANDLE FLAME
When hydrocarbons such as coal gas and natural
gas are burned, the flame has a very interesting, although
somewhat complicated, structure. A candle, composed of a mixture
of paraffin and stearic acid (both of which contain carbon and
hydrogen), makes an interesting subject for study.
The upper portion of a lighted candle, heated
by the flame above, melts so that the top becomes a cup holding a
small portion of the melted wax. This melted portion is drawn up
the wick by capillary action and vaporizing forms the first cone
of the candle flame. This cone is indicated in the diagram as the
inner cone -A-, the dark area immediately surrounding the
wick. If we should insert a tube
88 THE
STORY OF CARBON
into this portion of the flame, we could let
out some of the vapor which would condense to a solid similar to
the composition of the candle itself. This cone of the flame,
composed as it is of unburned gases, is not very warm and, in
fact, the head of a match could be placed there without igniting.
The second cone -B- in a candle flame, or the
intermediate cone, consists of vapors decomposed by the heat with
a small quantity of carbon which, being heated to incandescence,
makes the flame visible.
The third area or cone -C- is a practically
invisible narrow outer cone in which the hydrogen and carbon are
burned to water and carbon dioxide respectively.
EXPERIMENT No. 146 Water Forms When A Candle
Burns
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Glass and candle.
PROCEDURE:
Hold a cold, dry glass over a lighted candle with the mouth just
above the flame. Note the formation of moisture on the inner wall of
the glass.
SUMMARY:
The hydrogen from the candle reacts with the oxygen in the air to
form water.
EXPERIMENT No. 147 Lamp Black
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Candle and spoon.
PROCEDURE:
Light the candle. Hold a dry spoon in the flame above the wick for a
few seconds. Note whether any carbon deposits appear on the spoon.
SUMMARY:
The free carbon in the luminous zone will show its presence by
blackening the cold spoon. The cold object chills the flame so that
carbon does not burn completely. This very finely divided carbon is
known as lamp black.
EXPERIMENT No. 148 Lighting A Candle By Its Gases
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Candle with long wick.
PROCEDURE:
Light candle and let it burn for several seconds. Blow out the
flame. Wait a moment and then relight the candle by holding a match
a short distance above the wick.
SUMMARY:
When a candle is extinguished, gas briefly continues to issue from
the hot wick. If, during this time, a lighted match is held as
above, the gas will ignite. This experiment cannot be performed if
the candle is allowed to get cold.
LIONEL
CHEM-LAB 89
EXPERIMENT No. 149 Extracting Gas From A Candle
Flame
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Candle and glass tube.
PROCEDURE:
Hold one end of the glass tube directly above the wick of the
lighted candle. Slant the tube upwards. With a match, light the gas
coming out of other end of the tube.
SUMMARY:
The inner cone of the flame, composed as it is of combustible gases,
is the source of the inflammable gas conducted through the glass
tube.
CARBON GASES
In many parts of the world fuel gases, known
as natural gases, issue from the ground or may be obtained by
drilling wells. This natural gas is largely methane mixed with
other hydrocarbons and some nitrogen.
We have already mentioned the production of
coal gas carried on in retorts heated by coke. Coal gas also is
chiefly a mixture of hydrocarbons. It burns with a luminous flame
but is too expensive to be used commonly as a fuel. It has been
largely displaced by water gas.
Water gas, or illuminating gas, is essentially
a mixture of carbon monoxide and hydrogen. It is made by the
reduction of steam by heated carbon, in other words, by passing
steam over very hot coal or coke. Water gas is very
effective as a fuel since both the carbon monoxide and hydrogen
formed by this reaction burn with a very hot flame.
CARBON DIOXIDE AND CARBON MONOXIDE
Carbon dioxide and carbon monoxide are the
oxides of carbon, so similar in chemical formula and yet so
different in physical properties. The first puts out fire, the
second burns. The first is heavier than air, the second lighter
than air. Carbon dioxide is in our lungs continuously and is
exhaled with every breath yet carbon monoxide is the deadly poison
found in automobile exhaust fumes and coal-burning furnaces which
causes so many deaths by asphyxiation.
Each has a very simple formula. The one (carbon
dioxide) is CO2; the other (carbon monoxide) is CO.
Certainly these two formulas appear to be very similar but what a
big difference results from the fact that carbon dioxide has one
more atom of oxygen than carbon monoxide.
CARBON DIOXIDE
The common name for carbon dioxide is carbonic
acid gas. It is colorless and odorless and it is formed whenever
carbon or any fuel is burned. A popular name for it is "soda-pop"
gas.
90 THE
STORY OF CARBON
There is a small amount of carbon dioxide in
the atmosphere since it is essential to plant life. Some of
nature’s processes tend to increase the supply while other
processes tend to diminish it. For example, the processes which
tend to create carbon dioxide are respiration (breathing),
combustion, decay of organic material and volcanic action. When
people and animals breathe in oxygen from the air, it is taken up
by the blood stream, distributed to all parts of the body to
combine with carbon, and then is exhaled from the lungs as carbon
dioxide and water vapor. Another source of carbon dioxide is the
burning of fuel and here again the same action takes place: carbon
is oxidized to carbon dioxide. Other sources of this gas are to be
found in the atmosphere above volcanoes and certain man-made
industrial processes.
While these processes are creating carbon dioxide,
other processes at the same
time are using up the carbon dioxide which has been
formed. Plants growing in the sunlight absorb carbon dioxide
from the air utilizing the carbon and returning the oxygen to the
air. Various rock materials are continually taking carbon dioxide
out of the atmosphere and combining with it to form carbonates.
The action of the sea dissolves vast quantities of carbon dioxide
from the air, some of it eventually taking the form of sea shells
as calcium carbonate.
EXPERIMENT No. 150 Preparation Of Carbonic Acid
(CL-55, CL-66, CL-77)
APPARATUS:
Test tube, red litmus paper, blue litmus paper, sodium bicarbonate,
gas generator bottle, vinegar and funnel.
PROCEDURE:
Place a strip of red litmus paper and a strip of blue litmus paper
in a test tube half full of water. Place a half teaspoonful of
sodium bicarbonate in the gas generator bottle. Add enough water to
cover the sodium bicarbonate. Set up the gas generator apparatus,
making sure that the bottom of the funnel is below the surface of
the liquid. Extend the long end of the delivery tube into the test
tube containing the water and the litmus papers. Pour a few drops of
vinegar into the gas generator bottle. This liberates carbon dioxide
gas which passes into the test tube and changes the color of the
blue litmus paper to red. Remove the delivery tube from the test
tube and heat the water in the test tube until it boils. Drop a
small piece of blue litmus paper in the test tube and note that this
time there is no change of color.
SUMMARY:
Carbon dioxide combines with water to form carbonic acid which turns
blue litmus paper red. When the carbonic acid solution is heated the
carbon dioxide gas becomes less soluble and is driven off. Thus, the
solution is no longer acid and the blue litmus paper remains
unchanged.
To test for the presence of carbon dioxide in the following
experiments, a supply of limewater is required. Prepare this
limewater by adding one
LIONEL
CHEM-LAB 91
measure of calcium oxide to each of three test
tubes three-quarters full of water. Shake each test tube well to
allow the undissolved particles to settle out. Pour oil the clear
liquid into three clean test tubes and use as directed in the
following tests.
EXPERIMENT No. 151 Making Carbon Dioxide
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Limewater, sodium carbonate, two test tubes, tartaric acid, delivery
tube and stopper.
PROCEDURE:
Dissolve three measures of sodium bicarbonate in a test tube half
full of water. Add two measures of tartaric acid and promptly insert
the gas delivery tube. Extend the long stem of the delivery tube
into the limewater solution.
SUMMARY:
Sodium carbonate reacts with tartaric acid to liberate carbon
dioxide gas. This gas passes through the delivery tube into the
second test tube reacting with limewater to form insoluble calcium
carbonate which causes the solution to become milky.
FIGURE 15
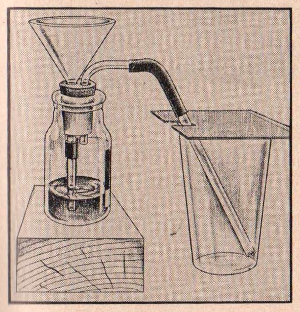
EXPERIMENT No. 152 Carbon Dioxide Will Not
Support Combustion
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bicarbonate, match, glass or tumbler, teaspoon, funnel,
stopper, delivery tube, vinegar, gas generator bottle.
PROCEDURE:
Place a heaping teaspoonful of sodium bicarbonate in the gas
generator bottle and add enough Water to cover the chemical. Insert
the stopper containing the funnel and delivery tube. Allow the long
stem of the delivery tube to go almost to the bottom of the tumbler
or glass and
place a piece of cardboard across the top, as shown in the
illustration. Now slowly pour some strong vinegar in the funnel of
the gas generator bottle. When the reaction appears to be
slowing down, add a little more vinegar. Remove cardboard from
tumbler and quickly insert a lighted match.
SUMMARY:
The lighted match will go out almost immediately proving that a
flame cannot burn in carbon dioxide gas.
EXPERIMENT No. 153 Carbon Dioxide Is Heavier Than
Air
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
92 THE
STORY OF CARBON
APPARATUS:
Milk bottle, candle, tumbler, sodium bicarbonate and teaspoon.
PROCEDURE:
Set candle firmly upright in a tumbler making certain that the wick
does not extend beyond the half-way mark of the tumbler. Light
the candle. Place two heaping teaspoonsful of sodium bicarbonate in
the milk bottle and add a half glass of vinegar. Pour the carbon
dioxide gas which is set free by this reaction onto the lighted
candle, taking care not to allow any of the liquid to spill out of
the bottle. Note that the flame is extinguished.
SUMMARY:
Carbon dioxide gas goes down into the glass displacing the air
around the candle and extinguishes the flame. This demonstrates that
carbon dioxide is heavier than air and does not support combustion.
EXPERIMENT No. 154 A Burning Candle Forms Carbon
Dioxide
(CL-11, CL-22, CL-33. CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Limewater, two glasses, candle, saucer and bottle.
PROCEDURE:
Place a candle firmly upright in the center of the saucer and add an
inch or two of water. Light the candle and invert the bottle over
it. When flame is extinguished turn the bottle upright and pour in
the clear limewater solution. Shake well and note the turbid
solution.
SUMMARY:
A burning candle gives off carbon dioxide, and water in the form of
water vapor. When the limewater becomes turbid, it proves that the
gas is carbon dioxide.
EXPERIMENT No. 155 Burning Alcohol To Obtain
Carbon Dioxide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Pan, alcohol lamp, bottle, limewater, two glasses.
PROCEDURE:
Place alcohol lamp in the center of a pan containing an inch of
water. Ignite lamp and invert the bottle over it. When flame is
extinguished, turn the bottle upright and pour in the clear
limewater solution. Shake well and note whether the solution becomes
turbid.
EXPERIMENT No. 156 Burning Wood To Obtain Carbon
Dioxide
(CL-11. CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sliver of wood, bottle, limewater, two glasses.
PROCEDURE:
Light the sliver of wood and place it in the bottle. Cover top of
bottle with cardboard. When flame is extinguished, remove the cover
and pour some clear limewater into the bottle.
EXPERIMENT No. 157 Burning Paper To Obtain Carbon
Dioxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Paper, jar, limewater, two drinking glasses, candle.
PROCEDURE:
Add three measures of calcium oxide to a glass half full of water
and stir. Allow the undissolved particles to settle out and
carefully pour off the clear liquid into another glass. Light the
paper
LIONEL
CHEM-LAB 93
and drop it into the bottle. Cover the bottle with cardboard. When
the flame is extinguished, remove the cover and pour in some clear
limewater. Shake well and note whether the solution becomes turbid.
EXPERIMENT No. 158 Carbon Dioxide In The Breath
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
A drinking glass and limewater.
PROCEDURE:
Insert the glass tubing into the limewater and blow through it. Note
the reaction.
EXPERIMENT No. 159 Extinguishing A Flame
(CL-55, CL-66, CL-77)
APPARATUS:
Candle, tumbler, vinegar, sodium bicarbonate, eye dropper and
tablespoon.
PROCEDURE:
Place a candle firmly upright in a tumbler making certain that the
wick does not extend beyond the halfway mark of the tumbler. Light
the candle. Now place a tablespoonful of sodium bicarbonate in the
bottom of the tumbler. Add a few drops of vinegar to the sodium
bicarbonate.
SUMMARY:
The flame begins to flicker and diminish in size as soon as the
carbon dioxide is formed and finally goes out altogether.
EXPERIMENT No. 160 Another Way To Prepare Carbon
Dioxide Gas
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Aluminum sulfate, sodium carbonate and two test tubes.
PROCEDURE:
Dissolve three measures of sodium carbonate in a test tube half full
of water. Dissolve two measures of aluminum sulfate in a second test
tube half full of water. Add a few drops of the sodium carbonate
solution to the second test tube.
SUMMARY:
The gas which is liberated is carbon dioxide and the thick white
precipitate formed by this reaction is aluminum hydroxide.
EXPERIMENT No. 161 Identifying The Gas From "Soda
Pop”
(CL-55, CL-66, CL-77)
APPARATUS:
Limewater, rubber stopper, delivery tube, a little ginger ale or
other carbonated beverage.
PROCEDURE:
Attach the gas delivery tube and stopper to the bottle of ginger ale
or other soda water being tested. Insert the other end of the
delivery tube in some limewater solution and test for the presence
of carbon dioxide gas.
EXPERIMENT No. 162 Why Bread Rises
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Flour, sodium bicarbonate (baking soda), heating spoon, candle or
alcohol lamp.
PROCEDURE:
Place six measures of sodium bicarbonate in a cup containing a
spoonful of flour. Add water, stirring constantly, until a good
dough is obtained. Heat a small amount of the dough over a flame.
94 THE
STORY OF CARBON
SUMMARY:
When sodium carbonate is heated, it decomposes and produces carbon
dioxide. As the gas bubbles through the dough, it causes the dough
to rise and become porous.
IMPORTANCE OF CARBON DIOXIDE
Until a few years ago, carbon dioxide was of
negligible importance commercially. It was used for no more
serious business than to charge soda water and, generated by yeast
or baking soda, to make bread rise.
Today, of first importance is carbon dioxide’s
use to combat tire. That red, tubular tank on the running board of
a police radio car contains carbon dioxide gas. Also you may have
seen banks of these cylinders on the crash trucks at air fields.
A pilot, who has to parachute from a burning
plane over water, inflates his life vest by pulling release cords
on two tiny carbon dioxide capsules (smaller than cigars) in his
vest. Formerly, when an airplane was forced down at sea, it sank
to the bottom before the pilot was able to get out, now flotation
bags are automatically inflated by carbon dioxide when the plane
hits water and save both the plane and the pilot.
The chief use of carbon dioxide gas is in
fighting fires and in this function it serves mankind well both in
times of peace and war.
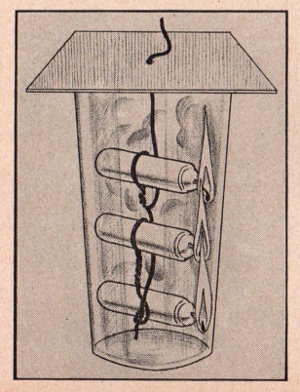
EXPERIMENT No. 163 An Experiment W1th Candles
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
FIGURE 16
APPARATUS:
Three small candles, wire, fruit jar and cardboard.
PROCEDURE:
Wire three candles together and suspend them in the jar as shown in
the illustration. Light the candles. Note that after burning a few
minutes the flames are extinguished.
SUMMARY:
The burning candles liberate carbon dioxide. Since this gas does not
support combustion, the candles slowly go out. The top candle goes
out first as the warm carbon dioxide gas rises to the top of the
bottle. When the gas finally reaches the bottom of the jar, the
other two candles also go out.
In line with our discussion of carbon
dioxide as a fire extinguisher, the following experiments on fire
control, using other chemicals, will be interesting.
LIONEL
CHEM-LAB 95
EXPERIMENT No. 164 Carbon Tetrachloride
Extinguishes Fire
(CL-66, CL-77)
APPARATUS:
Heating spoon, carbon tetrachloride, candle or alcohol lamp.
PROCEDURE:
Carefully heat a small amount of carbon tetrachloride in the heating
spoon. Light a match and hold it above the spoon in the fumes of the
liquid. Note that the flame is extinguished.
SUMMARY:
The vapor of the carbon tetrachloride will extinguish the flame
because it surrounds the object and prevents oxygen from reaching
it.
EXPERIMENT No. 165 How To Fireproof Cloth
(CL-11, LL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, candle or alcohol lamp, cotton cloth, test tube
and match.
PROCEDURE:
Dissolve fourteen measures of ammonium chloride in a test tube
one-third filled with water, using heat, if necessary, to hasten the
solution. Soak a piece of cotton cloth in the solution and then
allow the cloth to dry. Hold a lighted match to the cloth to see if
it will burn.
SUMMARY:
As in the case of carbon tetrachloride, ammonium chloride, when
heated, gives off fumes which smother flame. When heat is applied to
the ammonium chloride, a decomposition of the salt takes place,
resulting in ammonia gas and hydrochloric acid, neither of which
supports combustion.
EXPERIMENT No. 166 How To Fireproof Wood
(CL-11, CL~22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Match and sodium silicate solution.
PROCEDURE:
Dip the wooden part of a match stick in sodium silicate solution.
Light the match when the solution has dried and note that it is
extinguished as soon as it reaches the sodium silicate.
SUMMARY:
Sodium silicate is excellent for fireproofing wood since it has a
very high melting point and forms a protective coating which
prevents the oxygen from reaching the wood.
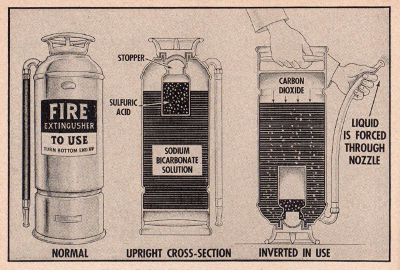
EXPERIMENT No. 167 A Dry Fire Extinguisher
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bicarbonate, tissue paper, tablespoon and tin plate.
96 THE
STORY OF CARBON
How a
Carbon Dioxide fire extinguisher works.
PROCEDURE:
Place a tablespoonful of baking soda on a small sheet of tissue
paper and fold the sides of the paper together. Put this on the tin
plate and ignite the paper.
SUMMARY:
Sodium bicarbonate when heated liberates carbon dioxide which
settles around the flame and smothers it.
EXPERIMENT No. 168 Making A Foam Fire
Extinguisher
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bicarbonate, aluminum sulfate, glass, two test tubes and
glue.
PROCEDURE:
Fill a test tube half full of a thin glue, then pour it into a
drinking glass. Add eight measures of sodium bicarbonate and stir
until a thin paste is formed. Add five measures of aluminum sulfate
to a test tube half full of water, shaking well to dissolve the
chemical. Pour this solution into the glass containing the thin
paste and note the foam which appears.
SUMMARY:
The foam formed in this reaction consists of carbon dioxide bubbles,
aluminum hydroxide, and the glue. It makes an excellent fire
extinguisher especially for burning gasoline and oil as the bubbles
of carbon dioxide surrounded by an emulsoid film adhere to the
burning surface better than free carbon dioxide.
LIONEL
CHEM-LAB 97
SOLID CARBON DIOXIDE
Carbon dioxide gas can not only be liquefied
but it can also be “frozen". It is then known as "dry ice" and is
familiar to every boy who has seen how a small piece placed in a
container of ice cream prevents the cream from melting and may
even freeze it as hard as stone.
Dry ice is made by purifying carbon dioxide gas
and passing it through charcoal to remove the odors. It is then
compressed and bottled in steel cylinders. This pure gas may be
made into “snow" by rapid expansion from the cylinder. The snow is
pressed and made into cakes which can be sawed apart with a band
saw. We must be very careful in handling solid carbon dioxide as
its freezing effect upon the tissues of the body is similar to a
severe burn.
CARBONATES
When carbon dioxide gas is bubbled through
water, some of it combines with the water to form the compound
carbonic acid. This is a very weak and unstable acid existing only
in water solution. However, it acts on bases to give solutions
called carbonates.
Some carbonates are stable solids found in
nature as rock formations, many of which are -of great value.
Limestone is mainly calcium carbonate while white marble is the
same chemically, but in crystal form and much purer. Ordinary
baking soda and washing soda are also carbonates known
respectively as sodium bicarbonate and sodium carbonate.
When a carbonate is treated with an acid,
carbon dioxide is given off as a gas. However, we will consider
many of the carbonates in more detail in other chapters of our
book when discussing Calcium and Sodium.
EXPERIMENT No. 169 Carbonate Test
(CL-G6, CL-77)
APPARATUS:
Limewater, sodium bisulfate, gas delivery tube and stopper, two test
tubes, small glass, mortar and pestle, and any chemical to which you
desire to apply the carbonate test.
PROCEDURE:
Prepare limewater as explained on page 91. Place five measures of
the substance to be tested in a test tube (grind if necessary). Add
five measures of sodium bisulfate and two or three drops of water.
Immediately attach the delivery tube and stopper with the long stem
of the delivery tube going into the test tube of limewater. Note
whether the limewater becomes turbid.
SUMMARY:
An acid will liberate carbon dioxide gas from a carbonate.
Consequently, by bubbling the gas into clear limewater and obtaining
a turbid solution, we know that the gas is carbon dioxide and the
compound a carbonate.
98
THE STORY OF CARBON
EXPERIMENT N0. 170 How A Carbonate Reacts With
Acids
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium carbonate, hydrochloric acid and test tube.
PROCEDURE:
Dissolve two measures of potassium carbonate in a test tube one-half
full of water. Add a few drops of hydrochloric acid. Continue adding
the acid until the fizzing stops. Pour the liquid into a saucer and
allow to evaporate. Note the residue.
SUMMARY:
Potassium carbonate reacts with hydrochloric acid to form potassium
chloride and carbonic acid. The latter is very unstable and
decomposes into water and carbon dioxide. Thus, by evaporating the
water, we are able to recover the potassium chloride in the form of
crystals. Compounds which liberate carbon dioxide gas in the
presence of an acid are carbonates.
EXPERIMENT No. 171 Calcium Carbonate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, calcium chloride, two test tubes.
PROCEDURE:
Dissolve three measures of sodium carbonate in a test tube half full
of water. Dissolve two measures of calcium chloride in a second test
tube half full of water. Pour into this a few drops of sodium
carbonate solution and note the formation of a heavy white
precipitate.
SUMMARY:
An exchange of elements occurs between the two compounds, resulting
in sodium chloride, or ordinary table salt, and the white
precipitate of calcium carbonate.
EXPERIMENT No. 172 Chromium Carbonate
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Chrome alum, sodium carbonate, two test tubes.
PROCEDURE:
Repeat Experiment No. 171 substituting chrome alum for calcium
chloride.
SUMMARY:
The bluish-green precipitate is chromium carbonate.
EXPERIMENT No. 173 Copper Carbonate
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, sodium carbonate and two test tubes.
PROCEDURE:
Repeat Experiment No. 171 substituting copper sulfate for calcium
chloride.
SUMMARY:
These compounds react to form sodium sulfate and the blue
precipitate of copper carbonate.
EXPERIMENT N0. 174 Manganese Carbonate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Manganese sulfate, sodium carbonate and two test tubes.
LIONEL
CHEM-LAB 99
PROCEDURE:
Repeat Experiment No. 171 substituting manganese sulfate for calcium
chloride.
SUMMARY:
The products formed in this reaction are sodium sulfate and the
white precipitate, manganese carbonate, which settles at the bottom
of the test tube.
EXPERIMENT No. 175 Ferrous Carbonate
(CL55, CL-66, CL-77)
APPARATUS:
Ferrous ammonium sulfate, sodium carbonate and two test tubes.
PROCEDURE:
Repeat Experiment No. 171 substituting ferrous ammonium sulfate for
calcium chloride.
SUMMARY:
The green precipitate formed as a result of this reaction is ferrous
carbonate.
EXPERIMENT No. 176 Magnesium Carbonate
(CL-66, CL-77)
APPARATUS:
Magnesium sulfate, sodium carbonate and two test tubes.
PROCEDURE:
Repeat Experiment No. 171 substituting magnesium sulfate for calcium
chloride.
SUMMARY:
When these two chemicals react in solution, they form the compound,
sodium sulfate, and the white precipitate, magnesium carbonate.
EXPERIMENT No. 177 Cobalt Carbonate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, sodium carbonate and two test tubes.
PROCEDURE:
Repeat Experiment No. 171 substituting cobalt chloride for calcium
chloride.
SUMMARY:
The products of this reaction are the soluble salt, sodium chloride,
and the insoluble cobalt carbonate which appears as a purplish-blue
precipitate.
EXPERIMENT No. 178 Strontium Carbonate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Strontium chloride, sodium carbonate and two test tubes.
PROCEDURE:
Repeat Experiment No. 171 substituting strontium chloride for
calcium chloride.
SUMMARY:
The interchange of elements in this case resulted in the formation
of the soluble salt, sodium chloride, and the white precipitate,
strontium carbonate.
100 THE
STORY OF CARBON
EXPERIMENT No. 179 Potassium Carbonate From Wood
Ash
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Wood ashes, tumbler, phenolphthalein solution, red litmus paper,
drinking glass and stirring rod.
PROCEDURE:
Place three spoonfuls of fresh wood ashes in a tumbler half full of
water and stir. Allow the undissolved particles to settle out and
then pour the clean solution into a glass. Dip a piece of red litmus
paper into the glass and note the color change. Add a few drops of
phenolphthalein.
SUMMARY:
Wood ashes contain potassium carbonate, a base which turns red
litmus paper blue and phenolphthalein, red.
EXPERIMENT No. 180 Precipitating Ferric Hydroxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, sodium carbonate and two test tubes.
PROCEDURE:
Repeat Experiment No. 171 substituting ferric ammonium sulfate for
calcium chloride.
SUMMARY:
The yellowish-brown precipitate is ferric hydroxide.
PETROLEUM AND GASOLINE
Most people think of the petroleum industry in
connection with the automobile and the airplane. But it is also
true that oil today flows through all the industrial arteries of
the world because of the magic of chemistry. It turns the wheels
or provides the lubrication for every machine that moves. It heats
many of our homes and buildings. It is the source of materials
that enter into the manufacture of countless commodities that play
a part in our everyday lives. The petroleum industry spends
$20,000,000 a year in research which has made all this possible.
In separating petroleum into its constituents
and processing them into substances which are vital to our
economic life, chemistry plays almost a magic part. It begins with
fractional distillation to separate the gases and light and heavy
liquids which comprise crude petroleum. It continues through the
new “cracking" processes which break down the heavier substances
and the polymerization processes which weld light gases into
liquids, which enable oil chemists to produce twice as much
gasoline from a barrel of oil as used to be possible. It makes
possible amazing processes whereby petroleum and its by-products
are metamorphosed into the countless products from alcohol to
chemical rubber which are rapidly finding their way into every
phase of modern life.
LIONEL
CHEM-LAB 101
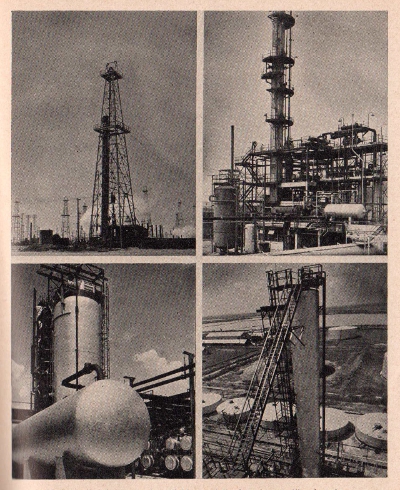
Modern
oil refining requires highly specialized equipment like that
shown on this page. This equipment used by the Texas Company
illustrates the processes from the derricks at the oil wells to
the stills and huge storage tanks where the refined oil is kept
until distribution.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook