The
Science Notebook
Lionel Chem-Lab
- Chapter 8
The
Science Notebook
Lionel Chem-Lab
- Chapter 8
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 121- 136
CHAPTER VIII
SULFUR
Sulfur is one of the most important elements
and also one of the most common. Physically, it is a pale-yellow
solid with no particular taste or odor.
There is a greater distribution of sulfur in
combination with other elements than in the pure state. For
example, there are numerous metallic ores which are sulfides: lead sulfide
(galena), zinc sulfide, and iron pyrites used in making sulfuric
acid. Many organic substances in the plant and animal world also
contain sulfur. Every boy recognizes hydrogen sulfide because it
has the disagreeable odor of rotten eggs.
You may recall also that certain foods like
eggs blacken silver spoons. This is due to the fact that sulfur is
present in these foods and reacts with the silver to form silver
sulfide, a black precipitate.
Sulfur is very valuable in the commercial and
industrial world being used in the manufacture of sulfuric acid,
many medicinal products, and to some extent for fireworks, matches
and gun powder.
HOW SULFUR IS MINED
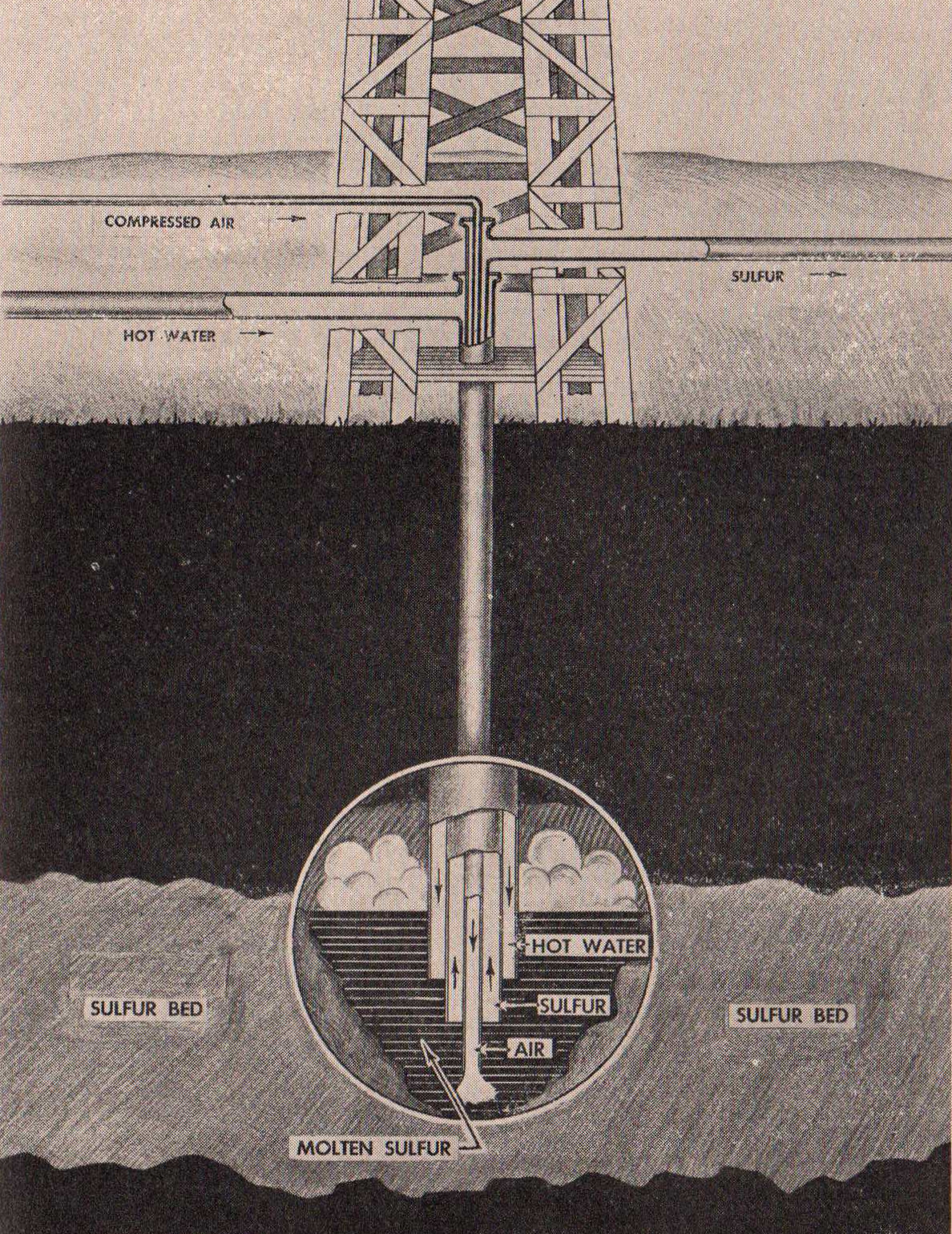
Sulfur occurs free in volcanic areas
impregnating the rocks. In Texas and Louisiana, the Frasch process
for the extraction of sulfur has been developed after many years
of experimentation amid the difficulties of quicksand and
poisonous gas. This process employs three concentric steel pipes
sunk four or five hundred feet below the surface of the ground
into the deposits. Superheated water is driven down through the
outside pipe and when it reaches the sulfur, melts it so that it
can be easily pumped to the surface. The melted sulfur then is
allowed to solidify into huge bins fifty feet high, built of
planks, and the solid sulfur forms a block which may contain as
much as 100,000 tons. For shipping, it is broken up by blasting
with dynamite. Sulfur mined in this fashion is so pure that for
general purposes it needs no refining.
FORMS OF SULFUR
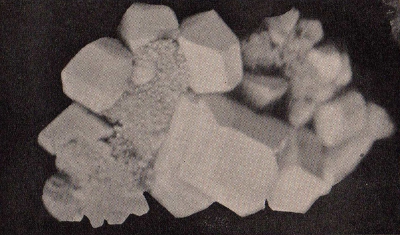
Solid sulfur occurs in three different
allotropic forms: rhombic,
prismatic (or monoclinic),
and amorphous (or plastic).
The large crystals shown in
121
122 SULFUR
the photograph are rhombic; the prismatic or
monoclinic variety has needle-shaped crystals while the amorphous
sulfur, as its name implies, is rubbery and non-crystalline.
These allotropic forms of sulfur exhibit widely varying physical
properties (color, shape, texture, solubility and stability) but
have identical chemical
properties.
Rhombic
Sulfur Crystals
EXPERIMENT No. 225 Making Elastic Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, test tube, alcohol lamp or candle.
PROCEDURE:
Place four measures of sulfur in a dry test tube. Heat quickly until
all the solid has melted and the color of the sulfur is changing.
Quickly pour the contents into a glass of water.
SUMMARY:
Note the elasticity of the sulfur due to the change in temperature.
EXPERIMENT No. 226 Allotropic Transformation Of
Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, candle or alcohol lamp, test tube.
PROCEDURE:
Place six measures of sulfur into a dry test tube. Cautiously apply
heat. Continue heating and note the various stages that the sulfur
passes through,
LIONEL
CHEM-LAB 123
Diagram
of the Frasch Process
124 SULFUR
SUMMARY:
Sulfur upon heating first melts to a pale yellow liquid which
gradually changes to a dark and viscous substance. Application of
more heat converts this into a vapor which condenses as it touches
the cool upper portions of the test tube.
EXPERIMENT No. 227 Preparation Of Monoclinic
Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, heating spoon, candle or alcohol lamp, paper.
PROCEDURE:
Place four measures of sulfur in your heating spoon. Slowly heat the
sulfur until it reaches the melting point, then remove from flame
immediately. Pour the liquid on a piece of paper. Examine crystals.
SUMMARY:
As explained above, sulfur exists in two solid forms: monoclinic and
rhombic. The monoclinic melts at 119 degrees and if cooled quickly
it will form needle-like crystals.
EXPERIMENT No. 228 Preparation Of Rhombic Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, heating spoon, glass, candle or alcohol lamp.
PROCEDURE:
Place four measures of sulfur in your heating spoon. Heat the sulfur
slowly. When the sulfur begins to melt, withdraw the spoon from
flame and pour contents into a glass of water. Examine the crystals.
SUMMARY:
Rhombic sulfur crystals are formed when sulfur is heated to 112
degrees. If monoclinic crystals are kept overnight they will form
rhombic crystals at room temperature.
EXPERIMENT No. 229 Plastic Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, heating spoon, glass and candle or alcohol lamp.
PROCEDURE:
Place five measures of sulfur in the heating spoon and apply heat
carefully until sulfur melts. Pour this into a glass of cold water.
Remove the sulfur and test it by touch. Set it aside and from time
to time note whether any changes occur.
SUMMARY:
When boiling sulfur is suddenly cooled by pouring it into cold
water, it assumes a rubbery, plastic form which is non-crystalline.
This is not a stable state because upon standing, the plastic sulfur
changes back into rhombic sulfur.
EXPERIMENT No. 230 A Chemical Property of Sulfur
(CL-44, CL-55, CL-66, CL-77)
LIONEL
CHEM-LAB 125
APPARATUS:
Sodium nitrate, sulfur, heating spoon, alcohol lamp or candle, gas
generator bottle, test tubes.
PROCEDURE:
Place three measures of sodium nitrate in the gas generator bottle.
Heat the contents thoroughly and collect the gas in two clean test
tubes. Reject the first test tube of gas collected as it is composed
largely of air which was present in your gas generator bottle.
Invert the second test tube to keep the gas from dissipating. Place
some sulfur in your heating spoon. Heat slowly unt1l the sulfur
begins to glow. Quickly drop the sulfur into the test tube
containing the gas.
SUMMARY:
Note how sulfur ignites in the presence of oxygen (produced when
sodium nitrate was heated).
EXPERIMENT No. 231 Sulfur Soluble In Carbon
Tetrachloride
(CL-66, CL-77)
APPARATUS:
Carbon tetrachloride, test tube, sulfur and saucer.
PROCEDURE:
Place a pin head amount of sulfur in a test tube containing half an
inch of carbon tetrachloride. Shake the test tube vigorously at
intervals and note whether the sulfur is dissolved. Pour the
contents into a saucer and allow the liquid to evaporate. Note the
sulfur residue on the saucer.
SUMMARY:
Sulfur is practically insoluble in water but readily so in carbon
tetrachloride.
EXPERIMENT No. 232 Making Sulfur From Two Gases
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfite, sodium bisulfate, test tube, paraffin, sulfur,
candle or alcohol lamp, gas delivery tube and stopper.
PROCEDURE:
Place three measures of sodium bisulfite and three measures of
sodium bisulfate into a test tube. Pour in a few drops of water and
inhale cautiously the odor given off. Put a small piece of paraffin
and four measures of sulfur into another test tube. Attach gas
delivery tube and stopper to the test tube containing the paraffin.
Heat gently. After noting the odor given off, run the long stem of
the delivery tube into the test tube containing the sodium
bisulfate. Note the sulfur formation when the two gases come in
contact with one another.
SUMMARY:
The gases formed are sulfur dioxide and hydrogen sulfide
respectively and when they are brought into contact with one another
they form the products, sulfur and water.
HYDROGEN SULFIDE
When sulfur combines with hydrogen, hydrogen sulfide, a colorless
gas with a disagreeable taste and odor is formed. A very poisonous
gas if inhaled in quantity, it produces headache, dizziness, and
nausea.
126 SULFUR
Hydrogen sulfide is a constituent of volcanic
gas and is sometimes found in spring water. It is generally a
product of decaying organic matter which contains sulfur. In the
laboratory, hydrogen sulfide is very important to the chemist
because by bubbling this gas through a salt solution, sulfides, the salts of
sulfuric acid are formed. Hydrogen sulfide decomposes easily and
has a strong tendency to combine with oxygen, thus it is a good
reducing agent.
EXPERIMENT No. 233 Preparation Of Hydrogen
Sulfide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Paraffin, sulfur, test tube and candle or alcohol lamp.
PROCEDURE:
Place a small piece of paraffin and four measures of sulfur in a
test tube. Heat gently for a minute or two. Remove test tube from
flame and note the gas which is given off.
SUMMARY:
When paraffin, a hydrocarbon, is heated, hydrogen is liberated which
reacts with sulfur to form hydrogen sulfide.
EXPERIMENT No. 234 Hydrogen Sulfide In
Illuminating Gas
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfide test paper, illuminating gas.
PROCEDURE:
Briefly open a gas jet and quickly expose some moistened sulfide
test paper to the gas. Turn jet off immediately. Note whether the
paper changes color.
SUMMARY:
If the illuminating gas contains hydrogen sulfide, a black spot will
appear on the moistened sulfide test paper.
EXPERIMENT No. 235 Another Way To Make Hydrogen
Sulfide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Iron sulfide, hydrochloric acid, sulfide test paper and test tube.
PROCEDURE:
Add a few drops of hydrochloric acid to a test tube containing one
measure of iron sulfide. Place some moistened sulfide test paper to
the mouth of the test tube and note the color.
SUMMARY:
The sulfur of the iron sulfide reacts with the hydrogen of the
hydrochloric acid to form hydrogen sulfide gas which turns the
moistened sulfide test paper a brownish-black color.
EXPERIMENT No. 236 Hydrogen Sulfide From Iron
Pyrites
(CL-66, CL-77)
APPARATUS:
Iron pyrites, hydrochloric acid, test tube, alcohol lamp or candle.
PROCEDURE:
Place a half measure of iron pyrites in a test tube. Add six drops
of hydrochloric acid.
LIONEL
CHEM-LAB 127
SUMMARY:
Note the odor of the gas formed which is hydrogen sulfide. The
explanation for this reaction is as follows: Iron pyrites, composed
of iron and sulfur reacts with hydrochloric acid to form iron
chloride and hydrogen sulfide.
EXPERIMENT N0. 237 Hydrogen Sulfide From Boiling
Cabbage
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfide test paper and cabbage.
PROCEDURE:
Place a strip of moistened sulfide test paper in the steam of
cooking cabbage. Note that a black spot appears on the paper.
SUMMARY:
Hydrogen sulfide, present in the cabbage, reacts with moistened
sulfide test paper turning it black.
EXPERIMENT No. 238 Acid Properties Of Hydrogen
Sulfide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Blue litmus paper, test tube, sulfur, paraffin, candle or alcohol
lamp.
PROCEDURE:
Place a small piece of paraffin and four measures of sulfur in a
test tube. Heat gently passing the gas through the delivery tube
into a test tube containing blue litmus paper and one inch of water.
Collect the gas in this tube and note that the litmus paper and
turns red.
SUMMARY:
Hydrogen sulfide solution turns blue litmus paper red proving that
the solution is acid.
EXPERIMENT No. 239 Testing For Hydrogen Sulfide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfide test paper, sulfur, paraffin, test tube, and candle or
alcohol lamp.
PROCEDURE:
Place a small piece of paraffin and four measures of sulfur in a
test tube. Heat gently for a minute or two. Insert a moistened piece
of sulfide test paper into the mouth of the tube and note the black
color reaction.
EXPERIMENT No. 240 Burning Hydrogen Sulfide
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Test tube, test tube holder, delivery tube and stopper, sulfur,
paraffin, drinking glass and candle or alcohol lamp.
PROCEDURE:
Place a small piece of paraffin and four measures of sulfur in a
test tube. Heat gently and insert the delivery tube and stopper.
Ignite the gas which escapes from the long stem of the delivery tube
and note that it burns with a pale blue flame. Place the side of a
cold glass in the blue flame and note whether any sulfur is
deposited on it.
128 SULFUR
SUMMARY:
Hydrogen sulfide burns with a pale blue flame in the presence of air
and the products formed are sulfur dioxide and water. However, when
the amount of air is limited it liberates free sulfur which settles
out as a yellow powder.
EXPERIMENT No. 241 Chlorine Reacts
With Hydrogen Sulfide
(CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, paraffin, gas delivery tube and stopper, test tubes,
tartaric acid, calcium hypochlorite and candle or alcohol lamp.
PROCEDURE:
Place a small piece of paraffin and four measures of sulfur in a
test tube. Insert the stopper and delivery tube with the long stem
going into another test tube one-quarter full of water and apply
heat gently. Allow the reaction to continue for awhile. Put two
measures of calcium hypochlorite and an equal amount of tartaric
acid in a test tube and add a little water. Transfer the delivery
tube and stopper to this test tube allowing the chlorine to bubble
into the hydrogen sulfide solution. Note the cloudiness of the
liquid.
SUMMARY:
When chlorine is passed through hydrogen sulfide solution, the
products formed are hydrochloric acid and sulfur. Some of the sulfur
settles as a white precipitate and some of it remains suspended in
the liquid causing the cloudiness.
OTHER SULFIDES
Sulfides can also be formed by the action of
hydrogen sulfide upon metals. Silver sulfide is the black tarnish
which appears on silverware due to the presence of small amounts
of hydrogen sulfide in the air and in illuminating gas.
EXPERIMENT No. 242 Tarnishing Metals With
Hydrogen Sulfide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL~77)
APPARATUS:
Sulfur, paraffin, test tube, candle or alcohol lamp and bright
silver coin.
PROCEDURE:
Place a small piece of paraffin and four measures of sulfur in a
test tube. Heat gently. As the gas escapes, hold the moistened
silver coin at the mouth of the test tube. Within a minute or so,
you will note that a black stain appears on the coin.
SUMMARY:
This black stain is silver sulfide formed when the hydrogen sulfide
gas reacts with the silver of the coin.
EXPERIMENT No. 243 Proving That Matches Contain
Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Safety matches, test tube, silver coin, candle or alcohol lamp.
LIONEL
CHEM-LAB 129
PROCEDURE:
Remove the heads of five safety matches and place them in a test
tube with several drops of water. Shake the tube until a thin paste
is formed. Place several drops of paste on the silver coin and heat
the coin until the paste begins to burn. Note the blackness of the
coin.
SUMMARY:
Silver sulfide was formed when the sulfur in the matches combined
with the silver of the coin.
EXPERIMENT No. 244 Another Silver Sulfide
Experiment
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, silver coin and paper.
PROCEDURE:
Place one measure of sulfur on a silver coin and wrap in several
layers of paper. Set aside for several minutes. Unfold the paper and
note the mark on the coin.
EXPERIMENT No. 245 Heating Iron Pyrites
(CL-66, CL-77)
APPARATUS:
Iron pyrites, candle or alcohol lamp and test tube.
PROCEDURE:
Place one measure of iron pyrites in a test tube. Heat gently. Note
the sulfur on the inside walls of test tube. Stop heating and
cautiously smell the odor coming from the tube.
SUMMARY:
Sulfur and iron react to form iron disulfide (iron pyrites) which
occurs abundantly in nature as yellow crystals, often called "fools
gold". When heated in a test tube, sulfur is liberated, most of
which is oxidized to the gas, sulfur dioxide.
EXPERIMENT No. 246 Distinguishing Pyrites From
Gold
(CL-66, CL-77)
APPARATUS:
Iron pyrites, hammer, glass, test tube, alcohol lamp or candle.
PROCEDURE:
Place one measure of iron pyrites in a test tube and heat carefully.
Note the sulfur formed on the inside walls of the test tube and the
strong odor given off. Carefully spread the material on a wooden
block. Pulverize the material with a hammer. Scratch a piece of
glass with a small piece of solid pyrites and note the marked
surface.
SUMMARY:
Compare these properties with the properties of gold. Gold does not
contain sulfide like iron pyrites, therefore, no decomposition
occurs when gold is heated, Gold is malleable, therefore, it can be
flattened out and not easily crushed to powder. Gold is soft,
therefore, it cannot scratch glass.
EXPERIMENT No.
247 Manganese Sulfide
(CL-33, CL-44, CL-55, CL-66, CL-77)
130 SULFUR
APPARATUS:
Manganese sulfate, test tube, sodium carbonate, sulfur, paraffin,
alcohol lamp or candle, stopper and delivery tube.
PROCEDURE:
Dissolve two measures of manganese sulfate in a test tube half full
of warm water. Add a measure of sodium carbonate and note the white
precipitate. Put a small piece of paraffin and four measures of
sulfur into another test tube and insert the delivery tube and
stopper. Heat and allow the gas to bubble into the tube containing
the precipitate. Note the pale pink precipitate.
SUMMARY:
The white precipitate which forms first is manganese carbonate.
Hydrogen sulfide changes the precipitate to manganese sulfide, a
pale pink substance.
EXPERIMENT No. 248 Nickel Sulfide
(CL-66, CL-77)
APPARATUS:
Nickle chloride, paraffin, sulfur, test tubes, candle or alcohol
lamp.
PROCEDURE:
Place four measures of sulfur and a small piece of paraffin in a
test tube. Heat gently for a few minutes. Dissolve two measures of
nickel chloride in a test tube half full of water. Introduce the
hydrogen sulfide into the solution. Note the black precipitate of
nickel sulfide.
EXPERIMENT No. 249 Testing Rubber For Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Rubber band, a penny, alcohol lamp or candle, heating spoon.
PROCEDURE:
Cut the rubber band into small pieces. Place the rubber and a bright
penny in the heating spoon. Heat slowly over a small flame. Stop
heating when the odor of burnt rubber is noticeable. Examine the
penny and note the black color.
SUMMARY:
The sulfur from the rubber reacts with the copper of the penny to
form copper sulfide.
EXPERIMENT No. 250 Reaction Of Copper And Sulfur
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, copper metal, test tube, candle or alcohol lamp.
PROCEDURE:
Put half a measure of sulfur on one end of the strip of copper. Hold
the strip with the test tube holder and heat until the sulfur melts.
Allow to cool and note the black film of copper sulfide left after
the sulfur is scraped off.
EXPERIMENT No. 251 Copper Sulfide Preparation
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, candle or alcohol lamp, sulfur, paraffin, test tube,
gas delivery tube and stopper.
LIONEL
CHEM-LAB 131
PROCEDURE:
Dissolve a few crystals of copper sulfate in a test tube one quarter
filled with water. Place a small piece of paraffin and four measures
of sulfur in another test tube. Insert the stopper and delivery tube
so that the long stem runs into the test tube containing the copper
sulfate solution and heat carefully. Note the formation of a black
precipitate of copper sulfide.
EXPERIMENT No. 252 Cobalt Sulfide
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, sodium carbonate, test tube, paraffin, sulfur,
alcohol lamp or candle, delivery tube and stopper.
PROCEDURE:
Dissolve a measure of cobalt chloride in a test tube half full of
water. Add a measure of sodium carbonate and note the blue
precipitate of cobalt carbonate. Put a small piece of paraffin and
four measures of sulfur into another test tube and insert the
delivery tube and stopper. Heat and allow the hydrogen sulfide gas
to pass into the precipitate.
SUMMARY:
Cobalt sulfide is formed by the action of the hydrogen sulfide on
cobalt carbonate.
EXPERIMENT No. 253 Zinc Sulfide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Zinc metal, sodium bisulfate, sulfur, paraffin, alcohol lamp or
candle, stopper and delivery tube, medicine dropper.
PROCEDURE:
Place a small piece of zinc and four measures of sodium bisulfate in
a test tube one third full of water. Heat until some of the zinc
dissolves. Pour off the clear solution into another test tube. Place
a small piece of paraffin and four measures of sulfur in a third
test tube. Attach delivery tube and stopper. Heat and allow the gas
to bubble into the zinc solution. Note the white precipitate of zinc
sulfide.
EXPERIMENT No. 254 The Reaction Between Sulfur
And Iron
CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, iron powder, heating spoon and alcohol lamp or candle.
PROCEDURE:
Mix one half measure of iron powder and two measures of sulfur on a
clean sheet of paper. Transfer them to the heating spoon and heat
slowly. Note the reaction between iron and sulfur to form ferric
sulfide.
EXPERIMENT No. 255 Ferrous Sulfide
(CL-55, CL-66, CL-77)
APPARATUS:
Ferrous ammonium sulfate, test tube, sodium carbonate, paraffin,
sulfur, delivery tube and stopper, candle or alcohol lamp.
132 SULFUR
PROCEDURE:
Place two measures of ferrous ammonium sulfate in a test tube half
filled with water and shake to dissolve. Add a measure of sodium
carbonate and note the green precipitate. Put a small piece of
paraffin and four measures of sulfur into another test tube. Fit the
delivery tube and stopper into the test tube and heat, allowing the
hydrogen sulfide gas to pass into the precipitate.
SUMMARY:
Sodium carbonate reacts with ferrous ammonium carbonate to form a
green precipitate, ferrous carbonate. When treated with hydrogen
sulfide gas, black ferrous sulfide is formed.
EXPERIMENT No. 256 Ferrous Sulfide From Sodium
Thiosulfate
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium thiosulfate, ferrous ammonium sulfate and sodium carbonate,
test tube, candle or alcohol lamp.
PROCEDURE:
Place one measure of sodium thiosulfate and an equal amount of
ferrous ammonium sulfate in a test tube half full of water and shake
to dissolve. Note the green precipitate formed when a measure of
sodium carbonate is added to this. Heat to boiling and note how the
color changes to black. In this case, the hydrogen sulfide gas is
formed from sodium thiosulfate.
EXPERIMENT No. 257 Hydrogen Sulfide From Sodium
Thiosulfate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium thiosulfate, sulfide test paper and test tube.
PROCEDURE:
Heat a test tube containing two measures of sodium thiosulfate. Note
the water which appears as the heating continues and the change in
the color of the solid. Remove from flame and cautiously smell the
odor given off. Insert a moistened piece of sulfide test paper in
the mouth of the tube and note the change in color denoting the
presence of hydrogen sulfide.
Sulfur dioxide and sulfur trioxide are two
well-known oxides of sulfur. When combined with water, they
form sulfuric acid and sulfurous acid, those tremendously
important acids used by the chemical industry.
SULFUR DIOXIDE
Sulfur dioxide is a stable, colorless gas with
a strong suffocating odor, almost twice as heavy as air, and very
soluble in water. In liquid form, for commercial use, it is
transported in tank cars.
Similar to hydrogen sulfide in that it is found
in volcanic gases and in sulfur spring waters, sulfur dioxide can
be prepared either by burning sulfur in the air or by the
reduction of sulfuric acid. Some of its important commercial uses
in addition to the making of sulfuric acid are for bleaching, the
preserving of foods, for disinfecting, and in place of ammonia as
a refrigerant.
LIONEL
CHEM-LAB 133
EXPERIMENT No. 258 Making Sulfur Dioxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS; Sulfur, candle or alcohol lamp and heating spoon.
PROCEDURE:
Place a measure of sulfur in the heating spoon. Heat carefully until
the sulfur melts and burns. Note the odor given off.
SUMMARY:
When sulfur is heated in air or oxygen, sulfur dioxide gas is formed
which can be recognized by its irritating odor.
EXPERIMENT No. 259 Chemical Properties Of Sulfur
Dioxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, red carnation or rose, alcohol lamp or candle.
PROCEDURE:
Place two measures of sulfur in a test tube. Heat the contents.
Moisten some carnation or rose petals and place at the mouth of the
test tube. Note how the sulfur dioxide gas bleaches the flower.
EXPERIMENT No. 260 Sulfur Dioxide From A
Bisulfite
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, sodium bisulfite, test tube and an eye dropper.
PROCEDURE:
Add four or five drops of water to a test tube containing two
measures of sodium bisulfite. Shake well and add two measure of
sodium bisulfate. Note the reaction. Smell cautiously the odor
coming from the mouth of the tube.
SUMMARY:
When sodium bisulfate is added to water, the liquid becomes acid.
Sodium bisulfite in the presence of this acid liberates sulfur
dioxide gas.
EXPERIMENT No. 261 Bleaching With Sulfur Dioxide
(CL-55, CL-66, CL-77)
APPARATUS:
Gas generator bottle, delivery tube and stopper, tumbler, colored
paper, sodium bisulfite, vinegar and funnel.
PROCEDURE:
Place the colored paper in the tumbler and add a few drops of water.
Put six measures of sodium bisulfite in the gas generator bottle and
pour in one half inch of water. Insert the stopper with the delivery
tube and funnel, the bottom of which must go below the surface of
the liquid. Place a piece of cardboard over the tumbler passing the
delivery tube into the glass through a hole in the cardboard. Now,
using the funnel, add some vinegar to the generator bottle. Note the
bleaching which occurs in the tumbler as the gas enters.
SUMMARY:
Sulfur dioxide, a good reducing agent, bleaches by removing oxygen
from colored substances. However, this bleaching is not permanent
because the color returns upon exposure to the air and sunlight.
134 SULFUR
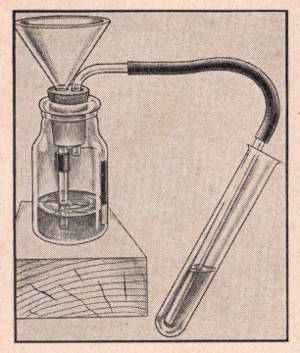
EXPERIMENT No. 262 Sulfurous Acid
(CL-55, CL-66, CL-77)
FIGURE 18
APPARATUS:
Sodium bisulfite, blue litmus paper, vinegar, gas generator bottle,
two hole stopper, funnel, delivery tube and sodium carbonate.
PROCEDURE:
Place six measures of sodium bisulfite into the gas generator bottle
and pour in about a half-inch of water. Insert the stopper with the
delivery tube and funnel. Make sure the bottom of the funnel is
below the surface of the liquid. Immerse a piece of blue litmus
paper in a test tube one-quarter full of water and allow the long
stem of the delivery tube to go into this. Now using the funnel add
some vinegar to the generator bottle. Note the reaction in the test
tube and also the color change of the litmus paper.
SUMMARY:
When the vinegar (an acid) was added to the sodium bisulfite, sulfur
dioxide gas was liberated and passed into the water with a bubbling
effect. Water and soluble sulfur dioxide react to form sulfurous
acid which turns blue litmus paper red.
SULFURIC ACID
Sulfur trioxide reacts readily with water to
form sulfuric acid. The two principal processes for making this
acid are the Lead Chamber
Process and the Contact
Process.
Concentrated sulfuric acid, commonly called oil of vitriol, is a strong
and corrosive liquid with oily characteristics. It is the most
important of all the acids commercially and industrially not only
because of its properties but also because it is inexpensive to
make. It has been said that, next to water, it is the liquid most
used by chemical industries. Some of its applications are in the
refining of petroleum, the manufacture of explosives, dyes,
hydrochloric and nitric acids and fertilizers.
EXPERIMENT No 263 How To Make Sulfuric Acid
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium nitrate, sulfur, test tube, blue litmus paper and alcohol
lamp or candle.
LIONEL
CHEM-LAB 135
PROCEDURE:
Mix one quarter measure of potassium nitrate thoroughly with one
quarter measure of sulfur and place in a test tube. Cautiously heat
contents and note the white vapors produced. Remove from flame when
the vapors stop. Put a cork in the test tube. Add enough water to
the test tube to fill it half full. Shake contents and drop in a
piece of blue litmus paper. Note the color change.
SUMMARY:
Sulfur is oxidized by the oxygen in the potassium nitrate to sulfur
trioxide. This compound forms sulfuric acid upon the addition of
water.
THE SULFATES
The salts of sulfuric acid are known as
sulfates, examples of which are iron sulfate, blue vitriol or
copper sulfate, and Epsom salt (magnesium sulfate).
Sodium thiosulfate, otherwise known as sodium
hyposulfite, has wide uses in photography. The photographer refers
to it as "hypo" and uses it to dissolve from the plate or film any
silver salts that have not been affected by light.
EXPERIMENT No. 264 Preparation Or Sodium Sulfate
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium chloride (table salt), sodium bisulfate, test tubes.
PROCEDURE:
Dissolve two measures of sodium chloride in a test tube half full of
water. In another test tube dissolve one measure of sodium
bisulfate. Gradually add the sodium bisulfate solution to the sodium
chloride solution. Note the white precipitate of sodium sulfate and
also the density of the gas which is liberated (hydrogen chloride).
EXPERIMENT No. 265 Reduction Of A Sulfate
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Charcoal block, sodium carbonate, sodium bisulfate, alcohol lamp,
blowpipe, sulfide test paper and an eye dropper.
PROCEDURE:
Make a small indentation in your charcoal block. Place in this a
mixture of one measure of sodium bisulfate with an equal amount of
sodium carbonate and one drop of water. Light the alcohol lamp and
direct the flame at this mixture with the blowpipe. Continue heating
until the substance melts. Set aside to cool. Remove the mass and
place it on a piece of moistened sulfide test paper. Note the stain
on the test paper.
SUMMARY:
When the sulfate reacts with carbon, and heat, it is reduced to a
sulfide which can be tested by placing it on a moistened piece of
sulfide test paper. The lead from the paper reacts with the
136 SULFUR
sulfide to form the black spot of lead sulfide. This is an excellent
test for the reduction of a sulfate.
EXPERIMENT No. 266 Strontium Sulfate Precipitate
(CL-66, CL-77)
APPARATUS:
Magnesium sulfate, strontium chloride and test tube.
PROCEDURE:
Place two measures of magnesium sulfate in a test tube one quarter
full of water and shake to dissolve. Put two measures of strontium
chloride into another test tube one quarter full of water and again
shake to dissolve. Pour the contents of one tube into the other and
note the heavy white precipitate. Add a few drops of hydrochloric
acid and note the precipitate still remains undissolved.
SUMMARY:
These compounds react to form magnesium chloride and insoluble
strontium sulfate which appears as a heavy white precipitate. This
sulfate along with those of lead and barium are the only sulfates
insoluble in water and hydrochloric acid.
EXPERIMENT No. 267 Iodine Test For Sodium
Thiosulfate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium thiosulfate, iodine starch test solution, test tubes,
hydrochloric acid.
PROCEDURE:
Dissolve two measures of sodium thiosulfate in a test tube half full
of water. Add five drops of hydrochloric acid. Cautiously heat the
solution. When cool, add four drops of iodine starch test solution
prepared as explained in Experiment No. 688. Note the blue color
formed.
SUMMARY:
Sodium thiosulfate when heated with hydrochloric acid liberates
sulfur dioxide which turns starch test solution blue.
EXPERIMENT No. 268 Reaction Of Thiosulfates
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Dilute hydrochloric acid, sodium thiosulfate, test tube, alcohol
lamp or candle.
PROCEDURE:
Dissolve two measures of sodium thiosulfate in a test tube half full
of water. Add eight drops of hydrochloric acid. Note turbidity. Heat
this solution.
SUMMARY:
Upon warming, sulfur dioxide is produced. The turbidity of the
solution is caused by the separation of sulfur and sulfurous acid.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook