The
Science Notebook
Lionel Chem-Lab
- Chapter 10
The
Science Notebook
Lionel Chem-Lab
- Chapter 10
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 141 - 148
CHAPTER X
PHOSPHORUS AND THE PHOSPHATES
The two principal forms of phosphorus are
white (or yellow) phosphorus and red phosphorus. The first is a
yellowish, waxy solid which is very poisonous and ignites so
readily that it must be handled under water. The red variety, on
the other hand, is neither poisonous nor easily ignitable. At
ordinary temperatures, white phosphorus slowly changes to red.
The chief use of phosphorus, both red and
white, is in the manufacture of matches. Safety matches, which
require a special surface in order to be ignited, are made of red
phosphorus. Regular matches are made of white phosphorus and
sulfur. White phosphorus is also used as a rat poison and in
making phosphoric acid. For military purposes, it is used to make
a smoke screen.
THE PHOSPHATES
The salts of phosphorus, known as phosphates, are important.
The most useful is calcium
phosphate, a mineral, which goes into the manufacture of
fertilizer.
EXPERIMENT No. 285 Manganese Phosphate
(CL-55, CL-66, CL-77)
APPARATUS:
Manganese sulfate, trisodium phosphate, two test tubes.
PROCEDURE:
Dissolve one measure of trisodium phosphate in a test tube half full
of water. Dissolve in a second test tube one quarter full of water
one half measure of manganese sulfate. Pour this solution into the
test tube containing the trisodium phosphate. Note the flesh-colored
precipitate of manganese phosphate.
EXPERIMENT No. 286 Preparation Or Magnesium
Phosphate
(CL-66, CL-77)
APPARATUS:
Magnesium sulfate, trisodium phosphate and two test tubes.
PROCEDURE:
Dissolve one measure of trisodium phosphate in a test tube half full
of water. Dissolve one measure of magnesium sulphate
141
142
PHOSPHORUS
in another test tube containing the same amount of water. Mix the
above solutions together. The white precipitate is magnesium
phosphate.
In most cases, whenever a dilute acid is added
to a phosphate, the phosphate dissolves completely, and a clear
solution remains.
EXPERIMENT N0. 287 Preparation Of Nickel
Phosphate
(CL-66, CL-77)
APPARATUS:
Nickel chloride, trisodium phosphate, two test tubes.
PROCEDURE:
Dissolve one measure of trisodium phosphate in a test tube half full
of water. Dissolve one measure of nickel chloride in a second test
tube containing an equal amount of water. The pale blue precipitate
is nickel phosphate.
EXPERIMENT N0. 288 Testing For The Solubility Of
Nickel Phosphate
(CL-66, CL-77)
APPARATUS:
Nickel chloride, trisodium phosphate, hydrochloric acid and two test
tubes.
PROCEDURE:
Prepare nickel phosphate as explained in the preceding experiment.
Add four drops of hydrochloric acid.
SUMMARY:
Note how the precipitate dissolves. The solution now consists of
nickel chloride and phosphoric acid.
EXPERIMENT No. 289 Preparation Of Manganese
Phosphate
(CL-55, CL-66, CL-77)
APPARATUS:
Manganese sulfate, trisodium phosphate and two test tubes.
PROCEDURE:
Place one measure of trisodium phosphate in a test tube half full of
water and shake to dissolve. Put two measures of manganese sulfate
in a second test tube half full of water and shake to dissolve. Use
heat if necessary. Allow solution to cool. When cool, add a few
drops of the trisodium phosphate solution and note the pinkish-white
precipitate of manganese phosphate.
EXPERIMENT No. 290 Precipitating Strontium
Phosphate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting strontium chloride for
manganese sulfate. The precipitate will be strontium phosphate.
EXPERIMENT No. 291 Preparing Ferrous Phosphate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting ferrous ammonium sulfate for
manganese sulfate. The greenish-white precipitate will be ferrous
phosphate.
LIONEL
CHEM-LAB 143
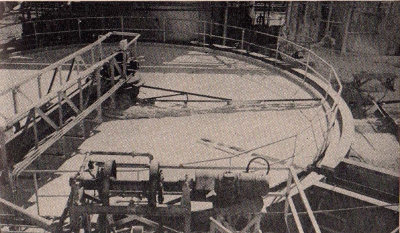
This
photograph shows one of the large vats at the American Potash
and Chemical Company plant in which sodium-lithium phosphate is
prepared.
EXPERIMENT No. 292 Preparation Of Ferric
Phosphate
(CL-66, CL-77)
Repeat Experiment No. 289 substituting ferric chloride for manganese
sulfate. The yellowish-white precipitate will be ferric phosphate.
EXPERIMENT No. 293 Precipitating Chromium
Phosphate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting chrome alum for manganese
sulfate. The precipitate will be chromium phosphate.
EXPERIMENT No. 294 Another Way To Make Ferric
Phosphate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting ferric ammonium sulfate for
manganese sulfate. The precipitate will be ferric phosphate.
EXPERIMENT No. 295 Precipitating Copper Phosphate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting copper sulfate for manganese
sulfate. The precipitate will be copper phosphate.
EXPERIMENT No. 296 Copper Phosphate Soluble In
Acid
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, trisodium phosphate, dilute hydrochloric acid and
two test tubes.
144 PH0SPHORUS
Repeat Experiment No. 295 and add a few drops of hydrochloric acid
to the precipitate. Note how readily it dissolves.
EXPERIMENT No. 297 Preparation
of Sodium Cobalt Phosphate
(CL-55, CL-66, CL-77)
APPARATUS: Cobalt chloride, trisodium
phosphate, charcoal bloch, pen knife, alcohol lamp, blowpipe.
PROCEDURE: Make a dent in the charcoal
block and place one quarter measure of trisodium phosphate in it.
Moisten the trisodium with a drop or so of cobalt chloride
solution. Direct the flame from the blowpipe directly on the
mixture. Note the blue mass formed when the cobalt reacts to form
cobalt phosphate.
EXPERIMENT No. 298 Precipitating Cobalt Phosphate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting cobalt chloride for manganese
sulfate. The blue precipitate will be cobalt phosphate.
EXPERIMENT No. 299 Precipitating Calcium Phosphate
( CL-55, CL-66, CL77)
Repeat Experiment No. 289 substituting calcium chloride for
manganese sulfate. The precipitate will be calcium phosphate.
EXPERIMENT No. 300 A Test for a Phosphate in Solution
(CL-77)
APPARATUS: Trisodium phosphate, ammonium
molybdate, hydrochloric acid, two test tubes.
PROCEDURE: Dissolve two measures of
trisodium phosphate in a test tube half full of water. Add two drops
of hydrochloric acid. Dissolve one measure of ammonium molybdate in
another test tube. Mix the two solutions. Note the yellow
precipitate of ammonium phosphomolybdate.
EXPERIMENT No. 301 Chromium Phosphate Soluble in Acid
(CL-55, CL-66, CL-77)
APPARATUS: Chrome alum, trisodium
phosphate, dilute hydrochloric acid and two test tubes.
PROCEDURE: Place one measure of trisodium
phosphate in a test tube half full of water and shake to
dissolve. Put two measures of chrome alum into another test
tube half full of water and shake to dissolve. Pour into this
a few drops of trisodium phosphate solution. Note the faint
blue precipitate which dissolves completely when the hydrochloric
acid is added.
LIONEL CHEM-LAB 145
EXPERIMENT No, 302 Precipitating Aluminum Phosphate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting aluminum sulfate for
manganese sulfate. The white precipitate will be aluminum phosphate.
EXPERIMENT No. 303 Preparation of Complex Salt
(C-L66, CL-47)
APPARATUS:
Nickel chloride, trisodium phosphate, ammonium hydroxide, two test
tubes.
PROCEDURE: Prepare a precipitate of
nickel phosphate as explained in Experiment No. 287. Add ammonium
hydroxide until the precipitate dissolves.
SUMMARY: Note color of the solution.
This solution contains a complex salt, that is, a compound of
nickel, ammonia and phosphorus. The chemical name is nickel ammonium
phosphate.
EXPERIMENT No. 304- Strontium Phosphate is Suluble in Acids
(CL-55, CL-66, CL47)
APPARATUS: Strontium chloride, trisodium
phosphate, hydrochloric acid and test tubes.
PROCEDURE: Prepare a precipitate of
strontium phosphate as explained in Experiment No. 290. Add some
hydrochloric acid. Note how an acid reacts with strontium phosphate
forming a weaker acid called phosphoric acid.
EXPERIMENT No. 305 Testing for Phosphates with Magnesium
(CL-47)
APPARATUS: Trisodium phosphate, magnesium
sulfate, ammonium hydroxide, ammonium chloride and test tubes.
PROCEDURE: Prepare some magnesium mixture
as described in Experiment No. 389. Dissolve one measure of
trisodium phosphate in a test tube half full of water. Add some
magnesium mixture to this solution. Note the crystalline
precipitate. Magnesium mixture is used to test for the presence of
phosphates in a solution.
EXPERIMENT No. 306 Another Way to Make Strontium Phosphate
( CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting strontium nitrate for
manganese sulfate. The precipitate will be strontium phosphate.
EXPERIMENT No. 307 Testing for a Base Reaction
(CL-55, CL-66, CL-77)
APPARATUS: Trisodium phosphate,
phenolphthalein, test tubes.
146
PHOSPHORUS
PROCEDURE:
Dissolve a measure of trisodium phosphate in a test tube half full
of water. Add two drops of phenolphthalein solution. Note the
reaction.
SUMMARY:
Since trisodium phosphate is composed of a weak acid (phosphoric
acid) and a strong base (sodium hydroxide) the solution will be
basic to the phenolphthalein solution (or form a pink color).
EXPERIMENT No. 308 Preparation Of Manganese
Ammonium Phosphate
(CL-77)
APPARATUS:
Ammonium hydroxide, trisodium phosphate, manganese sulfate and test
tubes.
PROCEDURE:
Prepare a precipitate of manganese phosphate as explained in
Experiment No. 285. Add some ammonium hydroxide. When ammonium
hydroxide is added to a precipitate of manganese phosphate, a
complex salt (manganese ammonium phosphate hydrated) is formed.
EXPERIMENT No. 309 Cobalt Phosphate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 289 substituting cobalt chloride for manganese
sulfate. The lavender blue precipitate will be cobalt phosphate.
EXPERIMENT No. 310 Solubility Of Magnesium
Phosphate
(CL-66, CL-77)
APPARATUS:
Trisodium phosphate, magnesium sulfate, hydrochloric acid and test
tubes.
PROCEDURE:
Prepare a precipitate of magnesium phosphate as explained in
Experiment No. 286. Add a few drops of hydrochloric acid and note
that the precipitate dissolves.
ANTIMONY
Antimony
is really a member of the nitrogen family along with phosphorus,
arsenic and bismuth. These elements are so classified because of
the similarity in their chemical behavior to nitrogen.
Antimony is a silvery, brittle solid having
certain properties common to metals and yet, in some respects it
is like a non-metal.
Stibnite,
supplied in Lionel Chem-Lab, is a widely-distributed sulfide
mineral which serves as the source for antimony. Commercially,
antimony is primarily valuable as an alloying metal. Because it
expands when it solidifies, it is used with lead and tin to make type metal. Thus it gives
printed letters a distinct and sharp appearance. Antimony is also
used in making Babbitt's metal,
a non-friction alloy.
LIONEL
CHEM-LAB 147
EXPERIMENT No. 311 Preparation Of Antimony
(CL-66, CL-77)
APPARATUS:
Stibnite, hydrochloric acid, zinc, test tube.
PROCEDURE:
Mix in a test tube half a measure of zinc, one measure of stibnite
and six drops of hydrochloric acid. The black precipitate is
antimony.
EXPERIMENT No. 312 Preparation Or Antimonyl
Chloride
(CL-66, CL-77)
APPARATUS:
Stibnite, hydrochloric acid, test tubes.
PROCEDURE:
Place one measure of stibnite and five drops of hydrochloric acid in
a test tube. Shake test tube well for a few minutes then pour the
liquid into a test tube three quarters full of water. Note the white
precipitate.
SUMMARY:
A solution of antimony in water forms a white precipitate, antimonyl
chloride.
EXPERIMENT No. 313 Testing Stibnite For Antimony
(CL-66, CL-77)
APPARATUS:
Stibnite, test tube and hydrochloric acid.
PROCEDURE:
Place a measure of stibnite in a test tube one quarter full of
water. Add four drops of hydrochloric acid. Shake test tube and note
color of solution.
SUMMARY:
If the solution forms an orange precipitate, antimony is present.
EXPERIMENT No. 314 Testing For Sulfide In
Stibnite
(CL-66, CL-77)
APPARATUS:
Stibnite, hydrochloric acid, test tube.
PROCEDURE:
Place one measure of stibnite and five drops of hydrochloric acid in
a test tube. Shake test tube well for about one minute. Cautiously
smell the odor in the test tube.
SUMMARY:
If the gas smells like rotten eggs, a sulfide is present in
stibnite. (Hydrochloric acid and stibnite made hydrogen sulfide
gas).
EXPERIMENT No. 315 Staining Silver With Stibnite
(CL-66, CL-77)
APPARATUS:
Stibnite, hydrochloric acid, silver coin and test tube.
PROCEDURE:
Place one measure of stibnite and six drops of hydrochloric acid in
a test tube. Place the silver coin at the mouth of the test tube for
a few minutes then remove it and note that a black coating is formed
on the coin.
148
PHOSPHORUS
SUMMARY:
Stibnite is a mineral consisting of antimony and sulfide, therefore,
upon the addition of hydrochloric acid the stibnite decomposes
forming antimony chloride and hydrogen sulfide. The hydrogen sulfide
reacts with the silver coin to form a black stain of silver sulfide.
EXPERIMENT No. 316 Staining Copper With Stibnite
(CL-66, CL-77)
APPARATUS:
Stibnite, hydrochloric acid, copper coin and test tube.
PROCEDURE:
Place one measure of stibnite and six drops of hydrochloric acid in
a test tube. Place the copper coin at the mouth of the test tube for
a few minutes then remove it and note that a black coating is formed
on the coin.
EXPERIMENT No. 317 Making An Iron Nail Black
(CL-66, CL-77)
APPARATUS: Iron nail, stibnite, hydrochloric
acid and test tube.
PROCEDURE:
Place one measure of stibnite, six drops of hydrochloric acid and a
clean iron nail in a test tube. Shake test tube for a minute then
remove nail and note the color. Stibnite and hydrochloric acid stain
iron black.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook