The
Science Notebook
Lionel Chem-Lab
- Chapter 17
The
Science Notebook
Lionel Chem-Lab
- Chapter 17
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 198 - 200
CHAPTER XVII
THE PRECIOUS METALS
Gold, silver and platinum are commonly referred
to as the precious metals. Gold is found in practically every part
of the world, usually in veins with quartz, or in alluvial
deposits. When found with quartz, it can be separated by
quarrying, crushing and by treatment with mercury. In alluvial
deposits it is extracted by the washing operation known as placer
mining.
As it is one of the heaviest of the metals and
not easily tarnished by exposure to the air, it is quite suitable
for use in coins. It is exceedingly ductile and malleable. For
example, gold not only can be beaten into extremely thin leaf but
a single grain of gold can be drawn into a wire five hundred feet
long.
SILVER
Silver is a heavy, soft, white metal, similar
to gold in that it is very ductile and malleable.
Silver has often been called the metal that
makes photography possible and about one hundred and fifty tons
are used annually in making photographic supplies. It is also used
in this country for making silver coins which contain 90% silver
and 10% copper. Silverware or tableware when made of sterling
silver contains 92% silver and 8% copper. Some silver is also used
in the manufacture of mirrors and electrical devices. Many
ornamental articles are made of cheaper metals plated with silver.
EXPERIMENT No. 480 How to Oxidize Silver
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium nitrate, test tube, candle or alcohol lamp, bright silver
coin.
PROCEDURE:
Put three measures of potassium nitrate in a test tube and heat
until crystals melt. Drop the coin in the test tube. Reheat test
tube for about a minute. Remove coin and note the formation of
silver oxide.
EXPERIMENT No. 481 Silver Sulfide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium thiosulfate, alcohol lamp, bright silver coin,
198
LIONEL
CHEM-LAB 199
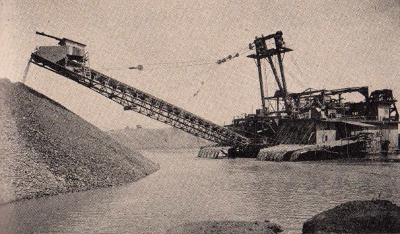
U. S.
Bureau Of Mines
This gold dredge
with a fifteen cubic foot capacity is a far cry from the pans of
the 19th century prospector.
test tube holder.
PROCEDURE:
Put one measure of sodium thiosulfate on a silver coin. Hold coin
with your test tube holder and heat carefully until the surface of
the coin darkens. Rinse the coin.
SUMMARY:
Heat causes the sodium thiosulfate to decompose liberating sulfur
which combines with silver to form black silver sulfide.
EXPERIMENT No. 482 Preparation Of Silver Chloride
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Hydrochloric acid, silver coin and eye dropper.
PROCEDURE:
Place a drop of hydrochloric acid on a silver coin. Set aside for
one hour. After an hour,
note the black stain which has formed.
SUMMARY:
The silver coin is stained black because silver chloride, formed in
the reaction between hydrochloric acid and silver, decomposes upon
exposure to sunlight.
PLATINUM
Platinum, the third of our precious metals, is
grayish-white and may be polished to a high luster. Like gold, it
is found in alluvial deposits.
Because platinum is a very inactive element
chemically and is not attacked by any of the common acids, the
dentist and the chemist find platinum
200
PRECIOUS METALS
instruments and containers invaluable in their
laboratories.
A good proportion of the demand for platinum is
found in the jewelry business where its attractive appearance, its
non-tarnishing features and its high cost make it desirable.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook