The
Science Notebook
Lionel Chem-Lab
- Chapter 2
The
Science Notebook
Lionel Chem-Lab
- Chapter 2
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 17 - 39
CHAPTER II
INTRODUCTION TO CHEMISTRY
Every one is acquainted with the most common
and useful kinds of matter. The water we drink, the food we
eat, the clothes we wear, the air we breathe; in short, anything
which has weight or occupies space can be referred to as matter.
Matter is composed of tiny particles called molecules which are the
smallest integral parts of a substance. A further
subdividing of these molecules will cause the substance to lose
its original properties.
DIVISION OF MATTER
Molecules cannot be divided
mechanically. There are more molecules in one drop of water
than there are drops in the Pacific Ocean. Take a match
stick, for example, and break it in half. Both pieces will
have the identical properties of wood, each piece consisting of
millions upon millions of molecules. Break the matchstick
again until the pieces are as small as you can get them and still
each fragment will be composed of millions of molecules each one
having the properties of the whole match stick.
To demonstrate further the division of matter, perform
the following experiment:
EXPERIMENT No. 1 Division Of Matter
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77) *
APPARATUS:
Sodium chloride (table salt) and three small glasses.
PROCEDURE:
Dissolve five measures of sodium chloride in a glass half filled
with water. Taste the solution. Divide the sodium
chloride solution equally among the three glasses. Fill the
these with water. Taste all solutions again.
SUMMARY:
Each solution has the salt taste and if the dilutions (or divisions)
or continued until the last drop, even here will the salt be
present.
LAW OF CONSERVATION OF MATTER
In addition to the above characteristics,
matter is a substance which cannot be created or destroyed.
This is commonly known as the law of Con-
* The numbers beneath the title of each
experiment indicate the Chem-Lab set with which the experiment
can be performed.
17
18
INTRODUCTION TO CHEMISTRY
servation of Matter and explains that,
although the matter may be broken up into small parts or
transformed as a result of many chemical actions, the one property
which can never be changed is its mass. That is to say, if
the weight of the materials used prior to the experiment is
compared with the weights of the resulting products, they will be
the same no matter how great the change may be.
EXPERIMENT No. 2 Law Of Conservation Of Matter
(CL-55, CL-66, CL-77)
APPARATUS:
Piece of wood and Lionel balance.
PROCEDURE:
Weigh piece of wood. Burn wood to ashes. Collect ashes
and weigh them.
SUMMARY:
The weight of the wood is less than it was before burning.
This apparent loss of weight, however, can be accounted for by the
gas and smoke which escaped into the air as the properties of the
wood changed while burning. If the gas and smoke could have
been weighed, they would account for the loss.
EXPERIMENT No. 3 Law Of Conservation Of Matter
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Candle, two dry wide-mouthed bottles, calcium oxide, test tube.
PROCEDURE:
Prepare limewater by dissolving four measures of calcium oxide in a
test tube filled with water. Cover and allow the undissolved
particles to settle to the bottom of the tub. Light
candle. Invert one bottle over the flame until flame goes out,
cover and remove. Pour clear limewater into the bottle and
shake gently. Add limewater to the other bottle and shake.
SUMMARY:
The flame of a burning candle seems to vanish giving off heat and
light while doing so. However, what we do not see is the
moisture being given off, part of which was collected on the cold
inside walls of the first bottle. As limewater is added to the
first bottle, the clear liquid becomes cloudy. This does not
happen in the second bottle which proves that the candle is not
destroyed but forms water and gas (carbon dioxide) as it burns
causing the milky appearance of the limewater.
STATES OF MATTER
When ice melts, it becomes water and this same
water eventually evaporates and becomes a gas. Thus, water
illustrates the three states in which matter exists - solid, liquid, and gas.
The states are dependent upon pressure and temperature.
LIONEL
CHEM-LAB 19
Matter is constantly in motion. This is
evident from the fact that a person sitting in a room adjoining a
kitchen where a gas jet is open will detect the odor.
Naturally, before one can smell the odor, it must be carried to
the nostrils. This carrying process illustrates matter in
motion.
EXPERIMENT No. 4 Matter In Motion
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Iodine crystals (obtainable at a drugstore), test tube and cork.
PROCEDURE:
Place one measure of iodine crystals in a dry test tube. Cork
the test tube. Apply heat slowly.
SUMMARY:
As the iodine crystals began to evaporate, or volatilize, a
beautiful violet colored gas forms and, in accordance with the
theory of the movement of matter, diffuses throughout the tube.
EXPERIMENT No. 5 Matter In Motion
(CL-11, CL-22, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride and a heating spoon.
PROCEDURE:
Place four measures of ammonium chloride on the heating spoon.
Heat for a few minutes. In a short time, the ammonia gas
liberated by decomposition will be easily detected by its pungent
odor.
Although it has been proved that molecular
movement occurs in all three states of matter, the motion is not a
visible one and can only be illustrated with gases and
liquids. The previous experiments illustrate this theory as
applied to gases while the following experiment will demonstrate
it in the case of liquids.
EXPERIMENT No. 6 Matter In Motion
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
Repeat Experiment No. 1
SUMMARY:
the taste of salt is in all three solutions proving that the
molecules of the liquid have distributed the salt to all parts of
the three glasses.
MOLECULAR THEORY
We have already mentioned that the three
classes of matter are gases, liquids, and solids. The
distinguishing physical properties of air, water and stone are
obvious, but only in recent years has science been able to
actually describe these differences in a satisfactory way by the Molecular Theory.
In the previous paragraphs, we mentioned that
all matter was composed
20
INTRODUCTION TO CHEMISTRY
of tiny particles called molecules which were
the smallest indivisible parts into which a substance can be
divided and still retain its original properties. In simple
language, this theory merely states that all matter has a granular
structure, somewhat similar, on a small scale, to a heap of
baseballs or a pile of sand.
Now the question arises as to the size of these
tiny particles. How large are they? Could they be seen
under a microscope? Science states that if ten million
molecules were placed in a row, there resulting length would only
be about the thickness of a dime. This gives you some idea
of their minuteness.
Another interesting fact about molecules is
that they are in constant motion and are striking and rebounding
against each other in ceaseless and tireless activity, somewhat
similar to the way particles of dust appear in a shaft of
sunlight. This leads up to the essential difference between
gases, liquids, and solids which can be explained entirely by the
movement and space occupied by the molecules. Science has
established that molecules of the same substance are always
exactly alike but their positions will vary considerably in
different states of matter.
For example, the difference between a gas and
liquid is that in the liquid the molecules are closer together and
their movements shorter. In both substances, however, the
molecules are in complete
disorder and always occupy a different spot having no
"home" or fixed place to return. But in solids, particularly
crystals, it appears that a molecule does not move out of its
prearranged course. If it did, the beautiful lines of a
crystal, it's faces, would ultimately change their size and shape.
CHANGES IN MATTER
If you were able to project yourself into the
world of a billion years ago to notice the plants, animals and
rocks of that ancient time, and then come back to make a
comparison with the matter of today, you would be forced to make
one conclusion: great changes in the world have taken place.
These changes have been chemical
and physical. That
is to say, the change may have been a reshaping of former
substances or formation of entirely new substances.
PHYSICAL AND CHEMICAL CHANGES
If an iron bar is heated to a high temperature
in a vacuum, it will become soft and melt, its color changing into
a dull red and eventually to a bright white. Interestingly
enough, however, if the metal is allowed to cool, the original
properties of the iron are restored. The heating produced a
change in physical properties but throughout the procedure the
iron remained iron. Thus it can be stated that a physical
change does not affect the composition of matter.
LIONEL
CHEM-LAB 21
Water freezes in cold weather to form ice
which melts with a rise in temperature and returns to its original
liquid state. Many of the properties of water were altered
when the water changed from liquid to solid, yet the substance is
still water.
Other familiar types of physical changes are
the melting of wax, the magnetizing of steel, dissolving of sugar,
crushing of stone and glass, and the changing of water into steam.
Thus, any change in a substance whereby some of
its properties are altered, but in which there is no change in the
composition of the substance, is called a physical change.
Expose an iron bar to moist air. In due
time it will rust into the reddish-brown powder which differs from
the iron in appearance. This is no longer the simple
substance of iron but a compound formed when the iron combined
with the oxygen in the air. Such a change is a chemical change and involves
the transformation of matter into a substance or substances having
entirely different properties.
Thus, we can say that a physical change is one
which leaves a substance unaffected while a chemical change is one
in which all the properties of the material are altered so that
one or more new substances are produced.
COMBINATION OR DIRECT UNION
Chemical reactions have been classified into
several types, the simplest form being combustion or direct union
wherein two or more substances combine to form a more complex
substance.
EXPERIMENT No. 7 Oxidation Of Iron By Heat
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered iron filings, heating spoon, alcohol lamp or candle.
PROCEDURE:
Place three measures of iron filings in the heating spoon.
Heat for a few minutes. Cool and examine.
SUMMARY:
The blue-black oxide formed by the direct union of iron with oxygen
in the air is the new compound, iron oxide.
EXPERIMENT No. 8 Oxidation Of Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, heating spoon, candle, or alcohol lamp.
PROCEDURE:
Place four measures of sulfur in the heating spending. Smell
the sulfur. Heat carefully until the sulfur begins to
burn. Smell the odor from the sulfur again, but this time very
cautiously.
22
INTRODUCTION TO CHEMISTRY
SUMMARY:
The sulfur did not give off any strong odor prior to heating.
With the application of heat, however, there was a direct union
between the sulfur and oxygen in the air to form the new product,
sulfur dioxide. This compound is easily detectable because of
its odor.
EXPERIMENT No. 9 Iron And Sulfur Chemically
Combined
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered iron, sulfur, test tube, candle or alcohol lamp.
PROCEDURE:
Mix thoroughly on a piece of paper five measures each of iron and
sulfur. Transfer mixture to a test tube. Heat for a few
minutes. Allow test tube to cool and examine contents.
SUMMARY:
The black porous, and non magnetic compound is ferrous sulfide the
properties of which are entirely different from those of iron and
sulfur. Since the iron and sulfur combined to form an entirely
new substance, it can be said that a chemical combination has
occurred.
EXPERIMENT No. 10 Zinc Combined With Sulfur
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered zinc, sulfur, heating spoon, candle or alcohol lamp.
PROCEDURE:
Mix one measure each of powdered zinc and sulfur on a piece of
paper. Place this mixture in the heating spoon and heat it
cautiously taking care to keep your face away from the reaction.
SUMMARY:
A flash reaction accompanies the chemical combination of zinc and
sulfur which forms zinc sulfide, often called zinc-blende or
blackjack by miners, and sphalerite by mineralogists.
EXPERIMENT No. 11 Carbon Combined With Oxygen
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Charcoal, heating spoon, candle or alcohol lamp.
PROCEDURE:
Place three measures of charcoal in the heating spoon. Heat
until the charcoal appears to turn gray.
SUMMARY:
When carbon or a substance containing carbon, such as charcoal,
wood, or coal is heated, the element combines directly with the
oxygen of the air to form carbon dioxide gas.
EXPERIMENT No. 12 Copper Combined with Oxygen
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Copper penny, test tube holder, alcohol lamp.
PROCEDURE:
Using the test tube holder, hold the face of a bright penny directly
over the oxidizing portion of the alcohol flame. Remove
LIONEL
CHEM-LAB 23
penny from the flame when scales began to form on the face.
After cooling, examine the scales.
SUMMARY:
Heating copper in the presence of air causes the formation of copper
oxide scales.
EXPERIMENT No. 13 Copper United With Sulfur
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Copper strip, sulfur, test tube, candle or alcohol lamp.
PROCEDURE:
Make some copper filings by scraping the copper strip with a course
file. Mix three measures each of copper and sulfur on a piece
of paper. Heat the mixture in a test tube. Cool and
examine contents.
SUMMARY:
Before heating, the sulfur and the copper filings were a simple
mixture. However, after heat has been applied, we obtain the
black mass of copper sulfide, the properties of which bear no
resemblance to those of the elements which it comprises.
EXPERIMENT No. 14 Magnesium Combined Directly
With Oxygen
(CL-77)
APPARATUS:
Powdered magnesium, heating spoon, candle or alcohol lamp.
PROCEDURE:
Place one quarter measure (and no more) of powdered magnesium in the
heating spoon. Apply heat cautiously, keeping your face away
from the reaction.
SUMMARY:
Magnesium combines so readily with oxygen in the air that a
brilliant flame accompanies the reaction. The white power,
magnesium oxide (commonly called magnesium) has properties
altogether different from the properties of its constituents.
Thus we know that the combining of the elements has resulted in a
chemical change.
DECOMPOSITION
Combination,
you recall, was a chemical change which had to do with putting
together substances to form a new one. Now let us study a
second type of chemical change, decomposition,
which is a separation or breaking down of a complex substance into
its constituent parts. The two reactions are direct
opposites. They can easily be remembered by thinking of the
first two letters of each word: co
meaning together and de meaning apart.
An electric current passed through water is an example of
decomposition. In this case, the products are hydrogen and
oxygen, the two constituent elements of water, and the process is
called electrolysis which
is explained to more clearly in our chapter on Water.
24
INTRODUCTION TO CHEMISTRY
EXPERIMENT No. 15 Decomposing Sugar
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sugar, dry test tube, candle or alcohol lamp.
PROCEDURE:
Place five measures of granulated sugar in test tube. Heat
slowly keeping the tube away from your face. Allow to
cool. Examine contents.
SUMMARY:
Heat decomposes the sugar which takes the form of water and a black
residue, mainly carbon, on the inner surface of the test tube.
EXPERIMENT No. 16 Decomposing Carbonate
(CL-77)
FIGURE 6
APPARATUS:
Marble chip (calcium carbonate), blowpipe, charcoal block and
alcohol lamp or candle.
PROCEDURE:
Embed a small marble chip in the charcoal block. Direct a
flame at the chip by means of the blowpipe. Heat for a few
minutes until red hot.
SUMMARY:
When heated the marble chip becomes whiter and when
LIONEL
CHEM-LAB 25
the flame is taken away, the white product left is calcium oxide,
often called lime. The gas given off in this reaction is carbon
dioxide.
EXPERIMENT No. 17 Decomposing Sodium
Thiosulphate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium thiosulfate, test tube, candle or alcohol lamp.
PROCEDURE:
Place four measures of sodium thiosulfate in the test tube. Heat
carefully for a few minutes. Test gas given off with sulfide (lead
acetate) test paper. Allow to cool. Examine contents.
SUMMARY:
By heating, sodium thiosulfate is decomposed into simple compounds
and elements. The first noticeable variation is the formation of
moisture on the inner walls of the test tube. This is the water of
crystallization of sodium thiosulfate which is being given off.
Moreover, the gas escaping from the mouth of the test tube is
hydrogen sulfide, easily detectable by its rotten egg odor and the
dark stain it forms on lead acetate test paper. As the heating
continues, sulfur deposits itself near the mouth of the test tube.
The other constituents which remain at the bottom of the test tube
are the compounds sodium sulfide and sodium sulfate.
EXPERIMENT No. 18 A Decomposition After An
Exchange
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, tartaric acid, two test tubes.
PROCEDURE:
Dissolve two measures of sodium carbonate in a test tube one-quarter
filled with water. Dissolve two measures of tartaric acid in a
second test tube containing the same amount of water. Pour the
contents of the second tube into the first. Note the bubbling at the
surface of the liquid.
SUMMARY:
Sodium carbonate reacts with tartaric acid to form sodium tartrate
and carbonic acid. This acid, being unstable, decomposes into water
and carbon dioxide gas. This gas causes the bubbling.
DOUBLE DECOMPOSITION
Still another type of chemical change is double decomposition in
which two compounds interact to form two other compound substances.
An interchange occurs, a re-pairing of chemicals much in the same
fashion that two dancing couples change partners.
EXPERIMENT No. 19 Interchange Of Elements In
Double Decomposition
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Strontium chloride, aluminum sulfate, two test tubes.
26
INTRODUCTION TO CHEMISTRY
PROCEDURE:
Dissolve three measures of strontium chloride in a test tube half
full of water. Using aluminum sulfate, repeat the procedure with the
other test tube. Mix the two solutions.
SUMMARY:
The elements of strontium and aluminum exchange places in the
reaction and the new compounds are aluminum chloride, a white
crystalline salt, and strontium sulfate, a heavy white precipitate
which settles at the bottom of the test tube.
EXPERIMENT No. 20 Another Double Decomposition
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, sodium ferrocyanide, two test tubes.
PROCEDURE:
Dissolve a small crystal of copper sulfate in a test tube half
filled with water. Repeat the procedure in the other test tube with
sodium ferrocyanide. Pour one solution into the other.
SUMMARY:
The elements of copper and sodium interchange positions and the new
products are sodium sulfate, a soluble salt, and copper ferrocyanide
which is insoluble and settles at the bottom of the test tube as a
reddish-brown precipitate.
EXPERIMENT No. 21 A Sulfate Reacts With
Strontium Nitrate
(CL-66, CL-77)
APPARATUS:
Aluminum sulfate, strontium nitrate and two test tubes.
PROCEDURE:
Dissolve two measures of aluminum sulfate in a test tube one-quarter
filled with water. Dissolve three measures of strontium nitrate in
another test tube one-quarter filled with water. Pour this into the
first solution.
SUMMARY:
The aluminum and the strontium exchange places to form aluminum
nitrate, which is soluble in water, and the white precipitate,
strontium sulfate.
EXPERIMENT No. 22 A Sulfate Reacts With Calcium
Oxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, calcium oxide and test tube.
PROCEDURE:
Dissolve one measure of ferric ammonium sulfate in a test tube half
filled with water. Add one measure of calcium oxide and shake the
tube well.
SUMMARY:
Ferric ammonium sulfate reacts with calcium oxide to form calcium
sulfate and the reddish-brown precipitate of ferric hydroxide.
DISPLACEMENT
The chemical change known as displacement, or simple
replacement, merely means that one element in a compound is
replaced by another free element.
LIONEL
CHEM-LAB 27
EXPERIMENT N0. 23 Chlorine Displaces Iodine
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, calcium hypochlorite, sodium iodide solution, test
tube.
PROCEDURE:
Dissolve one measure of sodium bisulfate in a test tube half filled
with water. Add a half measure of calcium hypochlorite. Shake well.
Add two or three drops of sodium iodide solution.
Shake again and observe the reddish-brown color.
SUMMARY:
Calcium hypochlorite reacts with sodium bisulfate forming chlorine
gas which remains dissolved in the solution. When the sodium iodide
is added, the chlorine takes the place of, or displaces, the iodine
forming sodium chloride (common salt) and liberating iodine
detectable by its brown color.
EXPERIMENT No. 24 Displacement Of Hydrogen By
Zinc
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Zinc, hydrochloric acid, test tube.
PROCEDURE:
Place a small piece of zinc in the test tube. Carefully add about
one third of a test tube full of hydrochloric acid. Observe the
rising of the hydrogen bubbles.
SUMMARY:
Acids, such as hydrochloric, contain hydrogen in combination with
other elements. Certain metals when brought into contact with these
acids displace the hydrogen in them. The zinc unites with the
chlorine of the hydrochloric acid to form zinc chloride and
hydrogen, some of which adheres to the zinc strip, or rises in the
form of bubbles to the surface.
EXPERIMENT No. 25 Zinc Displaces Copper From
Solution
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, zinc, test tube.
PROCEDURE:
Dissolve one small crystal of copper sulfate in a test tube
one-third filled with water. Drop into this a small piece of zinc.
Shake well and allow to stand for some time. Observe that the zinc
strip begins to take on the appearance of copper and the blue color
of the copper sulfate solution loses its intensity.
SUMMARY:
The zinc displaces the copper from the copper sulfate solution to
form the new compound, zinc sulfate, and the displaced copper
deposits itself on the zinc.
EXPERIMENT No. 26 Iron Displaces Copper From
Solution
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, test tube, powdered iron.
PROCEDURE:
Dissolve one small crystal of copper sulfate in a test tube
one-third filled with water. Add one measure of powdered iron to the
blue copper sulfate solution. Shake well for a few minutes. Note
28
INTRODUCTION TO CHEMISTRY
that the iron filings take on a rusty reddish appearance and the
blue solution becomes lighter.
SUMMARY:
Iron, in the form of iron filings, displaced the copper from the
copper sulfate solution and formed the compound known as iron
sulfate. The copper which is set free in the metallic state deposits
itself on the iron filings.
SUBSTANCES
The iron sash-weight of the window in your
room, the iron chain which holds the weight, the iron water pipes
in your cellar - forms so different to the manufacturers who made
them, are alike to the chemist, for they all consist of the same
substance - iron. A substance,
then, is a specific kind of matter, as iron, sugar or common table
salt. The same substance may occur in nature or in industry in a
variety of forms which appear to have little in common, yet to us
students in the field of chemistry, they are identical for they
all consist of a material having a definite set of characteristics
or properties.
Many naturally occurring materials are composed
of two or more substances which can be easily separated. These
specimens of matter are called complex
substances. Table salt is an example of a complex
substance since it can be separated chemically into sodium and
chlorine. If, however, a body is composed of only one substance,
it is said to be homogeneous,
meaning that it cannot be decomposed. Such a body is called a simple substance.
ELEMENTS
Ninety-two of these simple substances which do
not decompose are known to the chemists. These are the chemical elements, differing
from one another in physical properties and chemical behavior and
which can not be converted into simpler materials.
Every element is either metallic (iron, zinc,
copper, etc.) or non-metallic (carbon, iodine, and sulfur). The
word "metallic" makes us think of hard, heavy metals such as iron
and lead, but not all metals have these characteristics. The
metal, mercury, for example, is a liquid at ordinary temperatures;
the metals, sodium and potassium are very soft. There are,
nevertheless, common characteristics for these sixty odd metallic
elements. All of them are reflectors of light (polished silver
being the best), and all can conduct heat and electricity. Some of
them such as copper and silver are better conductors, than the
others. Also they are more or less malleable, that is, they can be beaten out under
a hammer and are more or less ductile,
which means that they can be drawn into wire. However, the degree
of malleability and ductility varies widely. Gold, for example,
can be easily hammered into sheets while antimony is so brittle
that it crumbles into a powder when struck a hard
LIONEL
CHEM-LAB 29
LIST OF ELEMENTS
|
Symbol |
|
Symbol |
Aluminum
Antimony
Argon
Arsenic
Barium
Beryllium
Bismuth
Boron
Bromine
Cadmium
Calcium
Carbon
Cerium
Cesium
Chlorine
Chromium
Cobalt
Columbium
Copper
Dysprosium
Erbium
Europium
Fluorine
Gadolinium
Gallium
Germanium
Gold
Hafnium
Helium
Holmium
Hydrogen
Indium
Iodine
Iridium
Iron
Krypton
Lanthanum
Lead
Lithium
Lutecium
Magnesium
Manganese
Mercury |
Al
Sb
A
As
Ba
Be
Bi
B
Br
Cd
Ca
C
Ce
Cs
Cl
Cr
Co
Cb
Cu
Dy
Er
Eu
F
Gd
Ga
Ge
Au
Hf
He
Ho
H
In
I
Ir
Fe
Kr
La
Pb
Li
Lu
Mg
Mn
Hg |
Molybdenum
Neodymium
Neon
Nickel
Nitrogen
Osmium
Oxygen
Palladium
Phosphorus
Platinum
Potassium
Praseodymium
Protactinium
Radium
Radon
Rhenium
Rhodium
Rubidium
Ruthenium
Samarium
Scandium
Selenium
Silicon
Silver
Sodium
Strontium
Sulfur
Tantalum
Tellurium
Terbium
Thallium
Thorium
Thulium
Tin
Titanium
Tungsten
Uranium
Vanadium
Xenon
Ytterbium
Yttrium
Zinc
Zirconium |
Mm
Nd
Ne
Ni
N
Os
O
Pd
P
Pt
K
Pr
Pa
Ra
Rn
Re
Rh
Rb
Ru
Sm
Sc
Se
Si
Ag
Na
Sr
S
Ta
Te
Tb
T1
Th
Tm
Sn
Ti
W
U
V
Xe
Yb
Y
Zn
Zr |
30
INTRODUCTION TO CHEMISTRY
blow. Copper may be drawn into a thin wire
while lead has neither ductility nor tensile strength.
The oxygen that you breathe, the iodine that you paint on your cut
finger, the sulfur that you burn when you strike a match - all
three are examples of non-metallic elements. However, the real
importance of this group of elements is their use as raw materials
which man converts into thousands of useful compounds.
COMPOUNDS AND MIXTURES
The elements sodium and chlorine combine
chemically to form common salt (sodium chloride), a chemical compound, which has
altogether different properties than either of its component
parts.
EXPERIMENT No. 27 A Chemical Compound
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Test tube, sulfur, powdered iron, candle or alcohol lamp.
PROCEDURE:
Repeat Experiment No. 9.
SUMMARY:
The new compound is iron sulfide or ferrous sulfide. It bears no
resemblance to either the original sulfur or iron. The name tells us
that iron and sulfur are constituents of the compound, iron sulfide.
The changing of properties through chemical
union and the necessity of new chemical reactions to decompose the
final product are guiding steps by which we can tell the
difference between a mixture and a compound. In a mixture, each of
the substances exhibits its own specific properties and
the term components of the
mixture is applied to the substances which make up the
mixture.
EXPERIMENT No. 28 A Mixture of Sand And Sugar
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sand and sugar.
PROCEDURE:
Mix three measures each of sugar and sand on a piece of paper. Taste
the mixture.
SUMMARY:
In a mixture, each component part maintains the same properties as
when it exists alone. By tasting, you can tell that the sugar is
still sugar and the sand is still sand.
EXPERIMENT No. 29 Separating Sand And Sugar
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sand, sugar, a drinking glass.
PROCEDURE:
Place three measures each of sand and sugar on a piece of paper. Mix
well and place in a drinking glass. Add water.
LIONEL
CHEM-LAB 31
SUMMARY:
By adding water, the sugar is dissolved and when the liquid is
poured off, the insoluble sand remains. Proof that it is sand alone
can be obtained by tasting. The sugar will return to its original
state if the water evaporates.
EXPERIMENT No. 30 Mixture Of Sand Powdered Iron
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, powdered iron and a toy magnet.
PROCEDURE:
Mix thoroughly three measures each of sulfur and powdered iron
on a sheet of paper. Separate into two equal parts. Pass the magnet
over one of the mixtures repeatedly. Place the other mixture in a
small glass and add water. Note that sulfur has a tendency to float
on the surface of the water, while the iron sinks to the bottom.
Carefully pour off the water thus removing some of the sulfur.
Repeat this procedure until only the iron remains in the glass.
SUMMARY:
Powdered iron and sulfur can be mixed so thoroughly that the
particles of each can only be distinguished with the use of a
microscope. Nevertheless, the separating of these two elements is
not difficult. Since iron is magnetic and sulfur is not, the iron
can be removed by passing the magnet over the mixture, or by the
addition of water, we can wash away the sulfur. Thus we have proved
once more that the components of a mixture remain the same and can
be separated.
ACIDS, BASES AND SALTS
We have seen that matter can be classified as
elements, compounds and mixtures. For purposes of study, we can
also subdivide compounds into three groups known as acids, bases, and salts.
Compounds known as acids derive their name from
the Latin word acidus,
meaning sour. All acids are compounds of hydrogen and another
element from which they derive their name. Thus, hydrochloric acid
is a compound of hydrogen and chlorine, sulfuric acid is a
compound of hydrogen, sulfur and oxygen, nitric acid is a compound
of hydrogen, nitrogen, and oxygen. Other common acids are citric
acid which gives the sour taste to oranges, lemons and grapefruit,
and acetic acid which is present in vinegar. Acids change blue
litmus paper to red and combine with many materials to form
compounds called salts.
EXPERIMENT No. 31 Characteristics Of Acids
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tartaric acid, glass stirring rod, small glass, blue litmus paper.
PROCEDURE:
Dissolve two measures of tartaric acid in a glass half
32
INTRODUCTION TO CHEMISTRY
filled with water. Place a drop on your tongue. Place a drop on some
blue litmus paper and observe the color change.
SUMMARY:
This experiment demonstrates that acids are sour and that they
change blue litmus paper to red.
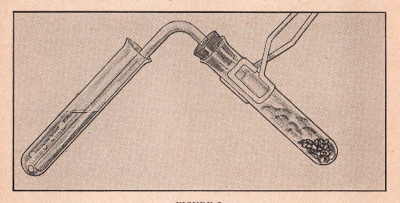
EXPERIMENT No. 32 Carbonic Acid
(CL-44, CL-55, CL-66, CL-77)
FIGURE 7
APPARATUS:
Sodium carbonate, sodium bisulfate, two test tubes, blue litmus
paper, bent tubing and stopper (delivery tube).
PROCEDURE:
Dissolve four measures of sodium carbonate and an equal amount of
sodium bisulfate in test tube half filled with water. When the
reaction starts, insert delivery tube and allow the long portion of
tube to go into the second test tube, one-third filled with
water. Allow the gas to bubble up through the water. Place a
few drops of the solution in the second test tube on your blue
litmus paper. Note color change.
SUMMARY:
Sodium carbonate, as we have noted in a previous experiment, gives
off carbon dioxide gas when treated with an acid. It is this gas
which causes the bubbling in the test tube. Moreover, the carbon
dioxide combines with the water in the tube forming carbonic acid.
This acid causes the blue litmus paper to turn red.
EXPERIMENT No. 33 An Acid From An Oxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, blue litmus paper, heating spoon, candle or alcohol lamp.
PROCEDURE:
Place two measures of sulfur in the heating spoon.
LIONEL
CHEM-LAB 33
Heat until sulfur melts. Hold a strip of moistened blue litmus paper
over the spoon. Note the change in the color of the paper.
SUMMARY:
The sulfur burns to form sulfur dioxide which reacts with the water
on the blue litmus paper to form sulfurous acid which turns the
litmus paper red.
EXPERIMENT No. 34 An Acid Produced By Double
Decomposition
(CL-11, CL-22, CL-33. CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, ammonium chloride, test tube, blue litmus paper,
candle.
PROCEDURE:
Place four measures of sodium bisulfate and three measures of
ammonium chloride in a test tube. Add a few drops of water. Heat
cautiously for a few seconds. Hold blue litmus paper over the mouth
of the tube. Note the color change.
SUMMARY:
Ammonium chloride reacts with sodium bisulfate, an acid salt, to
liberate hydrochloric acid fumes which turn blue litmus paper red.
BASES
Bases, the opposite of acids, turn red litmus
blue and are distinguished by the name hydroxide, that is, sodium hydroxide, potassium
hydroxide, calcium hydroxide, etc. Alkali is a term also used in referring to bases
of the metals.
The most important property of bases is
that, when combined with an acid, they neutralize each other and
lose their characteristic properties to form a compound called a salt.
EXPERIMENT No. 35 Properties Of Bases
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium hydroxide (or household ammonia), test tube, red litmus
paper.
PROCEDURE:
Add ten drops of ammonium hydroxide to a test tube half filled with
water. Place two or three drops of the solution on red litmus paper.
SUMMARY:
This solution, a base, turns red litmus paper blue.
EXPERIMENT No. 36 A Base From An Oxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, test tube, red litmus paper.
PROCEDURE:
Dissolve two measures of calcium oxide in a test tube half filled
with water. Place one or two drops of this solution on a small piece
of red litmus paper. Note the color change.
SUMMARY:
Calcium oxide in water forms a base, calcium hydroxide.
34
INTRODUCTION TO CHEMISTRY
EXPERIMENT No. 37 A Base Produced By Double
Decomposition
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, sodium carbonate, two test tubes, candle.
PROCEDURE:
Place three measures of calcium oxide and three measures of sodium
carbonate in a test tube one-quarter filled with water. Boil the
solution for a few minutes. Allow contents to cool. Pour off the
clear solution into another test tube. Place a few drops of the
solution on your finger tips and rub back and forth. Remove solution
from hands by washing immediately.
SUMMARY:
In this experiment calcium oxide combines with water to form calcium
hydroxide which in turn reacts with the sodium carbonate to form two
new compounds, calcium carbonate, the salt which settles at the
bottom of the tube, and sodium hydroxide, the base which gives a
soapy feeling to your hands.
SALTS AND NEUTRALIZATION
Our third classification is that of a salt.
The name given to the reaction occurring when an acid and base are
brought together to form a salt is neutralization.
For example, hydrochloric acid and sodium
hydroxide neutralize each other to form sodium chloride (table
salt) and water.
EXPERIMENT No. 38 Formation Of A Salt
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, test tube, acetic acid.
PROCEDURE:
Dissolve one measure of calcium oxide in a test tube one third
filled with water. Make the solution acid by dissolving in it one or
two drops of acetic acid. Note how clear the solution becomes.
SUMMARY:
Chemically speaking, a salt is a compound obtained by the
displacement of the hydrogen from an acid by the metal of a
base. In this experiment, the calcium oxide combines with
water to form calcium hydroxide. This base reacts with the acetic
acid forming water and the salt, calcium acetate. Since calcium
acetate is soluble in water, the solution becomes clear.
EXPERIMENT No. 39 Neutralization Of An Acid
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tartaric acid, phenolphthalein solution, ammonium hydroxide (or
household ammonia), test tube.
PROCEDURE:
Dissolve a measure of tartaric acid in a test tube half filled with
water. Add one drop of phenolphthalein solution. Add ammonium
hydroxide one drop at a time until the solution shows a faint pink
color. Continue adding one or two more drops of the ammonium
hydroxide, until the solution becomes a darker pink.
LIONEL
CHEM-LAB 35
SUMMARY:
In this experiment, ammonium hydroxide reacted with tartaric acid to
form water and ammonium tartrate, a base whose presence is detected
by the phenolphthalein which turns red in alkaline solutions.
Naturally, the more ammonium hydroxide is placed in the test tube,
the more alkaline the solution becomes and thus the more intense the
red indicator of phenolphthalein.
EXPERIMENT No. 40 Hydrolysis Of Ammonium
Chloride
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, test tube, blue litmus paper, candle or alcohol
lamp.
PROCEDURE:
Dissolve five measures of ammonium chloride in a test tube one
quarter filled with water. Heat slowly. Put a drop of this solution
on a piece of blue litmus paper.
SUMMARY:
Hydrolysis is the ability of water to break down a salt to form an
acid and a base. If the acid is stronger than the base, it will test
acid. If the reverse is true it will have a basic reaction. In this
case, ammonium chloride combined with water to form ammonium
hydroxide and hydrochloric acid. Since the acid is stronger, the
solution turns blue litmus red.
EXPERIMENT No. 41 Hydrolysis Of Sodium Carbonate
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, test tube, red litmus paper.
PROCEDURE:
Dissolve two measures of sodium carbonate in a test tube half filled
with water. Place one drop of this solution on a piece of red litmus
paper.
SUMMARY:
This is an example of hydrolysis giving an alkaline solution. The
sodium carbonate, a salt of a weak acid and a strong base, combines
with water to form carbonic acid and sodium hydroxide, the latter
being a very strong base giving the solution an alkaline reaction,
which turns the red paper blue.
EXPERIMENT No. 42 Hydrolysis Of Ferric Ammonium
Sulfate
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, three test tubes, candle or alcohol lamp.
PROCEDURE:
Dissolve two measures of ferric ammonium sulfate in a test tube one
quarter filled with water. Fill two other test tubes half full of
water. Heat one of them to boiling point. Add to both test tubes one
drop of ferric ammonium sulfate solution. Set the tubes in the test
tube rack. Note the orange precipitate in the test tube containing
the hot water.
SUMMARY:
Ferric ammonium sulfate undergoes hydrolysis in the presence of, or
mixed with, hot water. Ferric hydroxide is the orange
36
INTRODUCTION TO CHEMISTRY
colored precipitate while the clear liquid is sulfuric acid in
solution. The ammonium sulfate in the tube containing cold
water remains clear and unaltered.
EXPERIMENT No. 43 Interchange Of Elements
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, strontium chloride, two test tubes.
PROCEDURE:
Dissolve one measure of sodium carbonate in a test tube one quarter
filled with water. Dissolve in another test tube one measure of
strontium chloride in the same amount of water. Pour the strontium
chloride into the test tube containing the sodium carbonate and set
aside. Note the formation of a white precipitate at the bottom of
the tube.
SUMMARY:
The two compounds exchange elements forming sodium chloride, which
remains dissolved in the water, and strontium carbonate which
because of its insolubility settles at the bottom of the test tube.
LITMUS PAPER AND OTHER INDICATORS
We have mentioned that one of the
characteristics of acids is that it turns blue litmus paper red
and that bases turn the red back to blue. This is a common
chemical test to discover whether a given solution is acid or
basic. Of course, it is also used to decide whether or not a
solution is neutral because such solutions have no effect on
either blue or red litmus paper. Any material which changes color
in the presence of an acid or a base is known as an indicator. Indicating papers
used by chemists, such as red and blue litmus, are prepared by
soaking pieces of absorbent paper in the particular indicator
solution desired.
EXPERIMENT No. 44 A Solution Of Cochineal
(CL-77)
APPARATUS:
Cochineal, sodium carbonate, tartaric acid, test tube.
PROCEDURE:
Place one half measure of cochineal in a test tube one quarter
filled with water. Heat the solution until it becomes very red. Pour
off the clear solution into another test tube and add a half measure
of sodium carbonate. Now add one measure of tartaric acid and
observe all color changes.
SUMMARY:
Cochineal comes from the dried bodies of certain insects of Central
Mexico. It has the property of turning violet in the presence of
alkalies such as sodium carbonate, and a reddish-orange in the
presence of acids.
EXPERIMENT No. 45 Cochineal Paper
(CL-77)
LIONEL
CHEM-LAB 37
APPARATUS:
Cochineal, sodium carbonate, tartaric acid, test tube, filter paper,
candle or alcohol lamp.
PROCEDURE:
Place one measure of cochineal in test tube half filled with water.
Heat until the solution becomes red. Dip a strip of filter paper
into the solution and allow paper to dry. Test paper with a small
portion of moistened sodium carbonate. Repeat with moistened
tartaric acid. Note the color changes.
SUMMARY:
Cochineal paper can be used instead of cochineal solution when the
latter is less desirable. Both have the same property of changing
alkaline solutions violet and acid solutions reddish-orange.
EXPERIMENT No. 46 Logwood
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Logwood, two test tubes, sodium carbonate, tartaric acid.
PROCEDURE:
Place a small chip of logwood in a test tube half filled with water.
Heat until the liquid becomes deep red. Pour the clear red solution
into another test tube. Add one measure of sodium carbonate and two
measures of tartaric acid.
SUMMARY:
Logwood comes from the bark of a tree in the West Indies. Its
coloring matter has the property of turning reddish-blue in an
alkaline solution and reddish-brown in an acid solution.
EXPERIMENT No. 47 Logwood Paper
(CL-44, CL-55, CL-G6, CL-77)
APPARATUS:
Logwood, filter paper, sodium bisulfate, sodium carbonate, candle.
PROCEDURE:
Place a small chip of logwood in a test tube half filled with water.
Boil the liquid for a few minutes. Dip a strip of filter paper into
the liquid. Dry the paper and cut in two. On one piece put a half
measure of sodium carbonate. Sprinkle with water. Note the change in
color. On the other piece, place a half measure of sodium bisulfate.
Sprinkle with water. Note the color change.
SUMMARY:
The alkaline, sodium carbonate, turns the logwood paper reddish-blue
while the acid, sodium bisulfate, causes it to change to a
reddish-brown.
EXPERIMENT No. 48 Congo Red Paper
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Congo red paper, tartaric acid, sodium carbonate, small glass.
PROCEDURE:
Immerse a piece of congo red paper in a glass half filled with
water. Add two measures of tartaric acid. Stir and note change in
color. Now add three measures of sodium carbonate. Stir and again
note change in color.
38
INTRODUCTION TO CHEMISTRY
SUMMARY:
Congo red paper is made by staining white paper with a dye made from
coal tar. It has the opposite color effect of litmus, being red in
the presence of an alkaline solution and blue in an acid solution.
EXPERIMENT No. 49 Turmeric Paper
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, turmeric paper, tartaric acid, small glass.
PROCEDURE:
Dissolve two measures of sodium carbonate in a glass one-quarter
filled with water. Drop in a piece of turmeric paper. Stir and note
change. Now add four measures of tartaric acid. Stir and again note
change.
SUMMARY:
The coloring matter of turmeric paper comes from the root of a plant
of the Far East. It becomes brown in an alkaline solution and yellow
in acid solutions. Besides being used as a test paper, this pigment
is employed as a coloring agent for mustard.
EXPERIMENT No. 50 Litmus Paper
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Blue litmus paper, tartaric acid, sodium carbonate, red litmus
paper, small glass.
PROCEDURE:
Immerse a piece of blue litmus paper in a glass half full of water.
Add one measure of tartaric acid. Stir and note color change. Add
two measures of sodium carbonate. Stir and observe the change in
color.
SUMMARY:
When acid is added to water, blue litmus paper turns red and when
sodium carbonate is placed in the glass, the strip of litmus returns
to its original red color. Thus we can readily ascertain whether a
solution is acid, base or neutral by its action on litmus paper.
EXPERIMENT No. 51 Phenolphthalein
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Phenolphthalein solution, calcium oxide, tartaric acid, small glass.
PROCEDURE:
Put three drops of phenolphthalein solution in a glass half filled
with water. Add three measures of calcium oxide. Stir and note
change in color. Add three measures of tartaric acid. Stir and again
note color change.
SUMMARY:
Phenolphthalein turns red in the presence of bases, and colorless in
the presence of acids.
EXPERIMENT No. 52 Phenolphthalein Paper
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, phenolphthalein solution, filter paper.
LIONEL
CHEM-LAB 39
PROCEDURE:
Dip some filter paper into the phenolphthalein solution. Allow
to dry. Place a half measure of calcium oxide on this paper.
Sprinkle a few drops of water over it. Note the color change.
SUMMARY:
This is a handy method of testing with phenolphthalein when the
solution method is less desirable. Make some test paper to keep on
hand.
EXPERIMENT No. 53 Testing Laxatives
(CL-1, CL-2, CL-3 1/2, CL-5, CL-7 1/2, CL-10, CL-15)
APPARATUS:
Sodium carbonate, laxative tablet (from neighborhood drug store),
small glass.
PROCEDURE:
Cut tablet into small pieces and place in glass half filled with
water. Stir carefully for a few minutes and then add one measure of
sodium carbonate.
SUMMARY:
Some chewing-gum and candy laxatives contain phenolphthalein. The
presence of this substance will be indicated if the solution becomes
red after adding the sodium carbonate.
CAUSTIC ACIDS AND BASES
Certain acids and bases when full strength are
caustic poisons and must not be handled with the bare hands.
Examples of these are the acids hydrochloric, sulfuric, nitric and
acetic. Among the bases, sodium hydroxide and potassium hydroxide,
otherwise known as caustic soda and caustic potash, are also
dangerous to handle.
When these materials are supplied in Lionel
Chem-Lab sets or produced in any of the experiments, the solutions
are so weak and dilute that they are absolutely harmless.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook