The
Science Notebook
Lionel Chem-Lab
- Chapter 3
The
Science Notebook
Lionel Chem-Lab
- Chapter 3
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 40 - 57
CHAPTER III
THE ATMOSPHERE
Seldom do we realize that the entire earth is
covered with an ocean of gases called the atmosphere, which is
much deeper than the water that covers a large portion of the
earth’s surface. It has been estimated that the ocean of gases
comprising the atmosphere extends over one hundred miles in height
compared to the greatest ocean depth of six or seven miles.
Man’s physical structure is best adapted to work at the bottom of
this atmospheric ocean where the pressure is about fifteen pounds
to the square inch. Mountain climbers and aviators who ascend to
altitudes of more than four or five miles suffer because of the
lack of oxygen in the thin air and from the lower pressure and
frequently must carry oxygen tanks. Likewise deep sea divers
require oxygen to breathe when they descend to a deep level under
water.
COMPOSITION OF THE AIR
The air of the atmosphere, is composed of
numerous gases of which the principal ones are nitrogen and
oxygen, plus certain amounts of water and dust. We have already
seen that carbon dioxide is also present in the atmosphere but the
proportion of this gas to the other gases is very small indeed.
The remaining gases are known as rare gases and in recent years
have become of considerable commercial importance. These gases
include neon, krypton, argon and xenon.
OXYGEN
Oxygen is the most abundant of all the
elements and was discovered in England in the Eighteenth Century
by the chemist, Joseph Priestley. One day he put a small amount of
mercuric oxide in a tube filled with mercury, and then turned it
upside down in a vessel of mercury. When the oxide rose to the top
of the inverted tube, Priestley heated it with the rays of the sun
through a burning glass. He soon found that a gas was given off in
which a candle would burn with a remarkably brilliant flame. This
"air” discovered by Priestley was the gas we now call oxygen and
its discovery is considered one of the landmarks in the history of
chemistry.
40
LIONEL
CHEM-LAB 41
OCCURRENCE OF OXYGEN
Free oxygen is found in the atmosphere. It is
also found in many compounds comprising, for example, about 90% of
the weight of water. It makes up nearly one half of the rocks that
form the earth’s crust as well as over half of the plants and
animals including ourselves. Animal life is entirely dependent on
oxygen. When we inhale, we take oxygen into our lungs where it
passes into the blood stream. As the blood goes through our
circulatory system, oxygen is liberated. After it has been
utilized by the tissues to provide us with energy and warmth,
carbon dioxide is produced which is returned to the lungs by the
blood and finally exhaled. The important part that carbon dioxide
plays in this process is described more fully in our chapter on
Carbon.
EXPERIMENT No. 54 Release Of Oxygen by Leaves
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Bottle, green leaves, pan.
PROCEDURE:
Place some green leaves in a bottle. Fill the bottle to the brim
with water. Pour two inches of water into a pan. Place a dish over
the mouth of the bottle and invert it in the pan of water.
Remove the dish only after the mouth of the bottle is beneath the
water level of the pan. Expose bottle and pan to the sunlight and
allow to remain there for several hours.
SUMMARY:
This experiment indicates how cooperative nature is. We human
beings, together with the animals, breathe in oxygen to keep alive
and breathe out carbon dioxide gas as waste material. Conversely,
plant leaves absorb this gas, converting it to starch in the
presence of sunlight and releasing oxygen as waste. Actually what
has happened in this experiment is that the carbon dioxide in the
air is absorbed by the leaves leaving only oxygen and nitrogen. This
process tends to cause the water level to rise.
EXPERIMENT No. 55 Absorption Of Oxygen From
The Air
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered iron, wood chip, drinking glass, saucer.
PROCEDURE:
Fill the saucer with water and then place the wood chip in it. Place
four measures of powdered iron on the wood chip. Sprinkle a
little water over the filings. Invert the glass over the wood chip
so that the air is enclosed within. Set aside for a few days.
SUMMARY:
Note that after several days the water has risen slightly inside the
glass. This is due to the decrease in the amount of oxygen which has
united with the iron to form iron oxide.
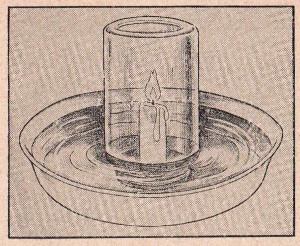
EXPERIMENT No. 56 Oxidation Of A Candle
(CL-11, CL-22, CL-33, CL-44. CL-55, CL-66, CL-77)
42 THE
ATMOSPHERE
FIGURE 8
APPARATUS:
Pan, candle, mason jar.
PROCEDURE:
Place candle firmly on a bit of melted wax in the center of the pan
and add two inches of water. Light the candle and place the inverted
glass jar over it making certain that the flame is a few inches away
from the top. Note that the flame goes out and the water levels
change.
SUMMARY:
The explanation of this interesting experiment is as follows: The
candle burns so long as there is oxygen. When this vital gas is used
up, the candle goes out. As the candle As the candle burns it forms
carbon dioxide and steam. However, since the carbon dioxide gas
dissolves in the water and the steam condenses into water, the water
in the pan rises to fill the space previously occupied by oxygen.
EXPERIMENT No. 57 Preparation Of Oxygen From
Potassium Nitrate
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium nitrate, test tube, candle or alcohol lamp and a cotton
string.
PROCEDURE:
Put three measures of potassium nitrate in a dry test tube and heat
until it begins to melt. Ignite the string and allow it to flame for
a moment. Blow the flame out and immediately insert the glowing
string into the test tube.
SUMMARY:
Potassium nitrate contains oxygen, part of which is liberated upon
heating. Since the air in the tube is richer in oxygen than the
surrounding air, the glowing string bursts into flame.
OXIDATION AND COMBUSTION
When oxygen combines with another element the
process of oxidation takes place. This process may be extremely
slow or extremely rapid. For example, the rusting of iron is an
illustration of slow oxidation, also the decay of organic matter.
No light is given off and practically no heat. On the other hand,
when a substance burns or undergoes combustion, oxidation is
taking place very rapidly and the heat which is given off may
cause the substance to glow like an ember or even to burst into
flame.
EXPERIMENT No. 58 Slow Oxidation
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered iron, tumbler.
LIONEL
CHEM-LAB 43
PROCEDURE:
Place three measures of powdered iron in a tumbler and add a few
drops of water. Set aside for a few hours and note the rust
formation.
SUMMARY:
Sometimes oxidation takes place so slowly that no light is seen and
unless careful measurements are made, no heat is noticed. This is an
example of slow oxidation.
Linde Air Products Co.
Heavy pieces of
metal are cut by the process of rapid oxidation. The metal is
heated white hot by a blowpipe, then a jet of oxygen blown
through a lance causes such rapid oxidation that the metal is
cut.
EXPERIMENT No. 59 Oxidizing Hydrogen Peroxide
(CL-55, CL-66, CL-77)
APPARATUS:
Fresh hydrogen peroxide (drug store), ferrous ammonium sulfate, test
tube.
PROCEDURE:
Dissolve two measures of ferrous ammonium sulfate in a test tube
half full of water. Add three drops of hydrogen peroxide. Note the
reaction and the color of the solution.
SUMMARY:
Hydrogen peroxide is a stronger oxidizing agent than free oxygen,
thus it oxidizes ferrous ammonium sulfate to ferric ammonium sulfate
(rust-colored solution).
EXPERIMENT No. 60 Fire Writing
(CL-11, CL-22, CL-33. CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium nitrate, candle or alcohol lamp, small soft brush and
paper.
44 THE
ATMOSPHERE
PROCEDURE:
Dissolve half a spoonful of potassium nitrate in a test tube
one-quarter filled with water. Use heat if necessary. Dip the brush
in the solution and write on the paper heavily in one continuous
line. Allow to dry. Light the end of the writing with a match to
start a spark on the paper, blowing out the flame. Note how the
spark moves along the line of the writing.
SUMMARY:
The ease with which potassium nitrate burns shows that it is
liberating oxygen rapidly as it burns along the written line.
EXPERIMENT No. 61 Making A Fuse
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium nitrate, cotton string and test tube.
PROCEDURE:
Dissolve ten measures of potassium nitrate in a test tube one
quarter filled with water. Dip the string into this solution for
several minutes. Allow to dry thoroughly by suspending it from a
hook.
SUMMARY:
Since potassium nitrate is easily combustible, it makes an excellent
fuse. Ignite and observe rapid travel of the combustion along the
string.
EXPERIMENT No. 62 Oxidation Of Paper
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium nitrate, test tube, alcohol lamp or candle, paper and test
tube.
PROCEDURE:
Place four measures of potassium nitrate in a test tube. Heat until
the crystals melt. Drop into the tube a few small shreds of paper
while continuing the heating.
SUMMARY:
When potassium nitrate is heated, it liberates oxygen which causes
the paper to burn.
SPONTANEOUS COMBUSTION
Occasionally we read in the papers of fires
which started by spontaneous combustion. What does this mean?
Sometimes among heaps of rags, wool and cotton, usually soaked
with oil; or wet hay or straw, a fire develops due to the internal
development of heat. Sometimes coal in the bunkers of vessels
catches fire in this same way. The name given to this phenomenon
is spontaneous combustion
which is the result of slow oxidation of combustible material in a
confined space where the small amount of heat liberated at first
cannot be dissipated. As the temperature rises, the oxidation
proceeds more rapidly until glowing, or even flaming, is produced.
LIONEL
CHEM-LAB 45
OXIDES
The name given to the compound formed when an
element combines with oxygen is an oxide. The burning of an element is really its
union with oxygen. When iron is burned in the presence of oxygen,
iron oxide is
formed. Likewise the burning of sulfur forms an oxide of
sulfur. Chemists, however, use the general term oxidation to refer
to the combining of many of the elements with one another even
though no oxygen happens to be involved in the reactions.
EXPERIMENT N0. 63 Zinc Oxide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Zinc metal, test tube, alcohol lamp.
PROCEDURE:
Place a small piece of zinc metal in a test tube and heat by means
of the alcohol lamp. Continue heating until the formation of a white
substance on the zinc appears.
SUMMARY:
The white coating is zinc oxide formed when the zinc undergoes
oxidation.
EXPERIMENT No. 64 Oxidation Of Sulfur By
Potassium Nitrate
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium nitrate, heating spoon, sulfur.
PROCEDURE:
Heat one measure of potassium nitrate on the spoon until it begins
to melt. Now add one measure of sulfur to the spoon. Note the
pungent odor.
SUMMARY:
Sulfur is quickly oxidized by potassium nitrate to form sulfur
dioxide recognizable by its penetrating odor.
EXPERIMENT No. 65 Oxidizing Hydrochloric Acid
(CL-77)
APPARATUS:
Sodium chromate, hydrochloric acid, and test tube.
PROCEDURE:
Place one measure of sodium chromate in a test tube and add ten
drops of hydrochloric acid.
SUMMARY:
Note the color of the solution. Sodium chromate oxidizes the
hydrochloric acid liberating chlorine gas which may be identified by
its sharp odor.
CAUTION:
While this gas is dangerous to inhale in large amounts, the amount
liberated in this case is quite negligible. However, it would be
well to avoid smelling the gas directly.
EXPERIMENT No. 66 Copper Oxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Clean copper wire, pliers, glass, gas flame.
46 THE
ATMOSPHERE
PROCEDURE:
Heat the wire over a gas flame holding it with a pair of pliers.
Continue this for a few minutes, noting the color of the flame, then
immerse the wire in a glass of water. Note the black formation.
SUMMARY:
This is another example of oxidation, the copper being oxidized to
form black copper oxide.
EXPERIMENT No. 67 Stannous Oxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tin metal, test tube, alcohol lamp.
PROCEDURE:
Put a small piece of tin into a test tube and apply heat by means of
the alcohol lamp. Continue heating until a white formation on the
tin may be noted.
SUMMARY:
The element tin is oxidized as it combines with the oxygen to form
the white coating of oxide which is a valuable abrasive used for
fine polishing.
EXPERIMENT No. 68 Iron Oxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Bright iron nail, test tube holder, alcohol lamp, glass.
PROCEDURE:
Heat the iron nail by holding it with the test tube holder in the
flame of the alcohol lamp. Continue this for a few minutes then
immerse the nail in a glass of water. Remove it when cool and note
any changes.
SUMMARY:
The black coating is due to the formation of iron oxide. Thus, when
iron is heated in the presence of oxygen, the iron is oxidized.
NITROGEN
Nitrogen is one of the four essential
constituents of air, there being about seventy-eight volumes of
nitrogen in every one hundred volumes of dry air.
It is also found in a number of compounds, principally potassium
nitrate (saltpeter), sodium nitrate (Chile saltpeter) and other
mineral nitrates which develop from the life processes of animal
and vegetable organisms. Large amounts of nitrogen exist in the
proteins in meat, egg white, clover, peas, beans, etc.
Like most of the other gases, nitrogen is colorless, tasteless and
odorless. On a large scale it is manufactured from liquid air. The usual method
of preparation in the laboratory is to make it from ordinary air
by removing the oxygen and making an oxide with some substance
that can then be easily separated from the nitrogen gas.
EXPERIMENT No. 69 Nitrogen Does Not Support
Combustion
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL~77)
LIONEL
CHEM-LAB 47
APPARATUS:
Wooden splint, bottle, candle, pan.
PROCEDURE:
Place a candle firmly upright in the center of a sauce pan. Fill
with two inches of water. Light the candle and place the inverted
bottle over it making sure that the flame is a few inches away from
the top of the bottle. Note that the flame is extinguished and the
water levels change. Remove the bottle and quickly insert a lighted
splint into the mouth of the bottle.
SUMMARY:
The lighted splint goes out proving that the nitrogen in the bottle
will not support combustion.
EXPERIMENT No. 70 Extracting Nitrogen From The
Air
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Candle, pan, bottle.
PROCEDURE:
Repeat Experiment No. 69 except when the flame goes out and the
water levels change, cover the mouth of the bottle so that the gas
cannot escape once the bottle is placed in an upright position.
SUMMARY:
The burning candle uses up oxygen, leaving essentially nitrogen in
the bottle.
IMPORTANCE OF NITROGEN
Nitrogen is vital to plant growth. A few
plants such as beans, peas, clover and alfalfa make direct use of
inactive nitrogen in the air with the aid of nitrogen-fixing
bacteria which are attached to their roots. These minute organisms
form many useful nitrogen compounds. Some of these are used by the
plants directly and the others stay in the ground to enrich the
soil. Although nitrogen is taken from the air by plants, it
is also returned to the air by the decay of plant and animal
matter. This is known as the nitrogen
cycle.
Nitrogen combines with hydrogen to form ammonia
and the chief use of nitrogen in industry is in the preparation of
this important substance - the first step in the manufacture of
important fertilizers and nitric acid. Nitrogen forms many
compounds which are unstable and break up with a release of energy
as, for example, tri-nitro toluene (TNT), ammonal,
nitro-glycerine, dynamite, etc. Huge plants have been erected by
the government for the purpose of fixing the nitrogen of the
atmosphere by means of electricity.
AMMONIA
Ammonia is composed of nitrogen and hydrogen
and has the formula NH3. It is a colorless gas
the water solution of which is known as ammonium hydroxide, or
ammonia water, commonly available at groceries and drug stores.
48 THE
ATMOSPHERE
The usual laboratory method of preparing
ammonia is by gently heating a mixture of ammonium chloride with
either calcium hydroxide or sodium hydroxide. The interaction of
these two compounds produces ammonium hydroxide, an unstable
compound, which decomposes into ammonia and water.
EXPERIMENT No. 71 Preparing Ammonia Gas
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, calcium oxide, test tube and red litmus paper.
PROCEDURE:
Place three measures of calcium oxide and an equal amount of
ammonium chloride in a test tube. Note whether an odor is given off.
Heat carefully. Remove test tube from flame. Carefully smell the gas
present. Moisten some red litmus paper and hold it over the mouth of
the test tube. Note the change in color.
SUMMARY:
In the laboratory, ammonia can be made by heating calcium oxide with
ammonium chloride to obtain ammonia, calcium chloride and water.
Since ammonia in the presence of water forms a base, it turns red
litmus paper blue.
EXPERIMENT No. 72 Extracting Ammonia From
Proteins
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Red litmus paper, calcium oxide, test tube, protein matter (white of
egg, cream, butter or gluten).
PROCEDURE:
Place a little protein matter in a test tube together with two
measures of calcium oxide. Add two or three drops of water and heat
gently. Smell the test tube cautiously to see if ammonia gas is
being liberated. Moisten some red litmus paper and place it over the
mouth of the tube.
SUMMARY:
Proteins are nitrogen compounds, many of which liberate ammonia gas
when heated with calcium oxide.
EXPERIMENT No. 73 Proving That Ammonia Is
Soluble In Water
(CL-33, CL-44, CL-55, CL-66, LL-77)
APPARATUS:
Calcium oxide, ammonium chloride, pan, test tube.
PROCEDURE:
Place two measures of ammonium chloride and three measures of
calcium oxide in a test tube. Heat tube and contents carefully
keeping the tube partly closed with the index finger. After
heating, place your finger tightly over the mouth of the tube.
Invert the test tube so that its mouth is beneath the water level of
the pan, taking your finger away only after the mouth of the tube is
entirely immersed in the water. Now note the rise of water in the
test tube.
SUMMARY:
Ammonia gas is soluble and is dissolved by water thus creating a
partial vacuum which causes the water to be sucked into the test
tube.
LIONEL
CHEM-LAB 49
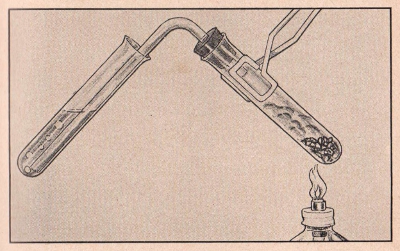
EXPERIMENT N0. 74 How To Make Aluminum Hydroxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
FIGURE 9
APPARATUS:
Calcium oxide, ammonium chloride, stopper and delivery tube, two
test tubes, holder, candle or alcohol lamp.
PROCEDURE:
Place three measures of ammonium chloride and an equal amount of
calcium oxide in a test tube. Attach delivery tube and heat slowly
holding the test tube with the holder. As the reaction develops and
gas begins to escape, extend the long stem of the delivery tube into
another test tube one fourth filled with water. Continue heating for
a few minutes and then remove test tube containing the water and the
gas. (Remove delivery tube from
receiving test tube before cooling the delivery test tube to avoid
sucking liquid back into a hot test tube and consequently breaking
the glass.) Dip a piece of red litmus paper into this
solution and observe the color change.
SUMMARY:
Heating calcium oxide and ammonium chloride liberates ammonia gas.
Since ammonia gas is soluble in water, it combines with water to
form the base, ammonium hydroxide, which turns red litmus paper
blue.
EXPERIMENT No. 75 How To Make Copper Ammonia
Blue
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, ammonium hydroxide (or household ammonia), test
tube.
50 THE
ATMOSPHERE
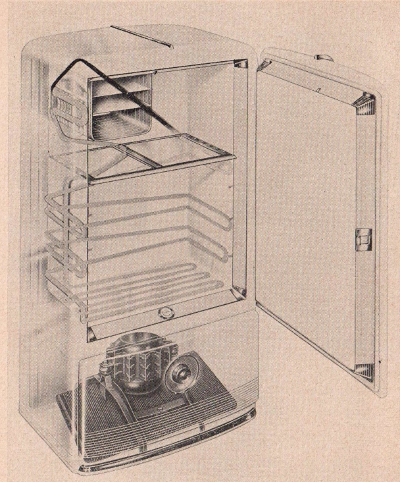
Frigidaire Div. G. M. C.
An illustration in
"phantom" of a modern electric refrigerator showing the motor
compressor unit, refrigeration coils, and ice unit. The
compressor converts the liquid refrigerant to a volatile gas
which is circulated through the coils, lowering the temperature
in the refrigerator.
LIONEL
CHEM-LAB 51
PROCEDURE:
Dissolve three measures of copper sulfate in a test tube one third
filled with water. Add a few drops of ammonium hydroxide and note a
bluish-white precipitate. Add more ammonium hydroxide and note the
deep blue color which appears.
SUMMARY:
The bluish-white precipitate is copper hydroxide. When more ammonia
is added, the copper hydroxide dissolves to form the beautifully
colored solution of copper ammonia blue.
EXPERIMENT No. 76 Characteristics Of Ammonium
Salts
(CL-66, CL-77)
APPARATUS:
Magnesium sulfate, ammonium hydroxide (or household ammonia),
ammonium chloride, two test tubes.
PROCEDURE:
Dissolve (by shaking well) two measures of magnesium sulfate in a
test tube one quarter filled with water. Add a few drops of ammonium
hydroxide. Note the cloudy appearance when the tube is again shaken.
Prepare an ammonium chloride solution by dissolving two measures of
ammonium chloride in a test tube one quarter filled with water. Pour
the contents of this tube into the first tube. Note that the
resulting solution is clear.
SUMMARY:
In the presence of excess ammonium salts, the precipitate of
magnesium hydroxide dissolves because the ammonium salts have
decreased the amount of hydroxide.
EXPERIMENT No. 77 Vaporizing Ammonium Chloride
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, test tube, candle or alcohol lamp.
PROCEDURE:
Place two measures of ammonium chloride in a dry test tube and apply
heat slowly. Note how the vapor condenses near the mouth of the
tube.
SUMMARY:
Many ammonium salts, including ammonium chloride, are vaporized
directly from a solid to a gas.
USES OF AMMONIA
Because of its great industrial importance,
large quantities of ammonia are required. It is manufactured
largely by either the Haber process which depends upon the
formation of ammonia by the direct union of nitrogen and hydrogen,
or from soft coal by the process known as destructive
distillation.
One of the most important uses of ammonia is as a refrigerant.
When a gas such as ammonia is liquefied, heat is liberated, and
likewise when the liquid passes again into the gaseous state, a
great deal of heat is absorbed. This fact is utilized in the
manufacture of ice and in maintaining a low temperature in cold
storage plants.
52 THE
ATMOSPHERE
Other uses for ammonia are: to make household
ammonia, to make ammonium salts (fertilizers and explosives), to
make nitric acid and to make sodium carbonate (washing soda) by
the Solvay Process.
MODERN ELECTRIC REFRIGERATORS
Ammonia was quite satisfactory for the
commercial manufacture of ice and certain other industrial
applications, but with the advent of air-conditioning and domestic
refrigerators, a new refrigerant was required which was not
poisonous, explosive, or inflammable. It must also have no odor
when mixed with air, even in fairly high concentrations, so that
panic would not result in crowded theaters or department stores
should a leak occur in the air-conditioning system. Applied
science, consequently, has synthesized a new family of
refrigerants fulfilling every requirement. These new materials
which are
fluorinated-chlorinated hydrocarbons, are safe, and
because of their safety are now widely used not only in domestic
refrigerators but in the air-conditioning of theaters, office
buildings, and a rapidly increasing number of homes.
NITRIC ACID
Nitric acid is the most common acid of
nitrogen and its chief use is in the making of explosives, such
as, nitro-glycerine, dynamite, gun cotton and TNT. It also is used
in the manufacture of fertilizers, artificial silk, drugs, dyes
and celluloid and to make such important nitrates as silver
nitrate employed in the manufacture of photographic film.
Nitric acid is very strong and reacts with
bases to form salts called nitrates.
EXPERIMENT No. 78 Preparing Nitric Acid
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Potassium nitrate, sodium bisulfate, test tube, blue litmus paper,
alcohol lamp or candle.
PROCEDURE:
Place four measures of potassium nitrate in a test tube. Add
four measures of sodium bisulfate and four or five drops of water.
Put a strip of moistened blue litmus paper over the mouth of the
test tube and apply heat. Remove from flame when fumes are beginning
to be given off and smell cautiously. Note that the litmus paper
turns red.
SUMMARY:
Nitric acid is manufactured commercially by treating saltpeter with
sulfuric acid. In the laboratory, however, it is made by the
reaction of potassium nitrate with sodium bisulfate.
FIXATION OF NITROGEN
The name given to the process of utilizing
free nitrogen in the air to make useful compounds such as nitric
acid, nitrates and ammonia, is the fixation of
LIONEL
CHEM-LAB 53
nitrogen.
Since the supply of natural nitrates such as Chile saltpeter
(sodium nitrate) is limited, and the demand for nitrogen and its
compounds is large, it becomes necessary to draw on the vast
resources of the air as a source.
Nearly half of the world’s requirements of nitrogen compounds are
fulfilled by artificial fixation processes: (1) the electric arc process, (2) the
synthetic ammonia process
and (3) the cyanamide process.
HYDROGEN
Hydrogen gas, the lightest of all elements,
was used for a good many years to inflate balloons and dirigibles
but because of its inflammability is no longer used for this
purpose. The most important use of hydrogen, today, is in the
treatment of certain oils to produce fats for household purposes.
This is known as the
hydrogenation process. Huge quantities of hydrogen are
also used in the manufacture of acids, ammonia and in metal
refining.
PROPERTIES OF HYDROGEN
Hydrogen is a gas without color, taste or odor
and does not occur in such quantities as oxygen and nitrogen. It
differs from oxygen in that only very small amounts of it occur in
the free state. The compounds of hydrogen, on the other hand, are
everywhere about us: in water, in foods and in our bodies. It
combines with carbon in all proportions to make hundreds of
compounds including petroleum and natural gases. These are known
as hydrocarbons.
We have already described the process of
oxidation. Hydrogen is a very important reducing agent because of its tendency to
combine with oxygen and thus remove oxygen from the oxides. The
process of reduction is
more fully explained on a following page.
Because hydrogen is a component of all acids,
it is sometimes known as an "acid-forming element".
The usual method of preparing this gas in the
laboratory is by using a metal such as zinc, and sulfuric or
hydrochloric acid.
EXPERIMENT No. 79 Hydrogen From Iron
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, copper sulfate, powdered iron, test tube, candle
or alcohol lamp.
PROCEDURE:
Dissolve five measures of sodium bisulfate and one half measure of
copper sulfate in a test tube one quarter filled with water. Heat
slowly, then add one measure of powdered iron.
SUMMARY:
Iron frees hydrogen from sodium bisulfate. The products are ferrous
sulfate and free hydrogen. As in the previous experiment, the copper
sulfate acts as a catalyst.
54 THE
ATMOSPHERE
EXPERIMENT No. 80 Hydrogen From Zinc
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, zinc, test tube, candle or alcohol lamp, copper
sulfate, wooden splint.
PROCEDURE:
Dissolve four measures of sodium bisulfate and one half measure of
copper sulfate in a test tube one quarter filled with water. Add two
or three small pieces of zinc. Heat carefully and note the reaction.
Place a lighted wooden splint at the mouth of the test tube.
SUMMARY:
Zinc displaces hydrogen from sodium bisulfate to form zinc sulfate
and free hydrogen. (The sodium bisulfate dissolved in water forms
dilute sulfuric acid.) The copper sulfate in this experiment serves
to hasten the reaction and is called a catalytic agent in the
language of chemistry.
FIGURE 10
EXPERIMENT No. 81 Test for Hydrogen Peroxide
(CL-77)
APPARATUS:
Sodium chromate, drinking glass, hydrogen peroxide solution (drug
store), hydrochloric acid, test tube, and stirring rod.
PROCEDURE:
Dissolve one half measure of sodium chromate in a test tube one
quarter filled with water and add four drops of hydrochloric acid.
Note the light orange color. Pour this solution into a drinking
glass and add five drops of hydrogen peroxide and stir. Note
that the solution becomes blue and then rapidly becomes light brown.
SUMMARY:
This test is often made to demonstrate the presence of hydrogen
peroxide in rain water.
HELIUM AND OTHER RARE GASES
Chemists studied the atmosphere for a great
many years before the elements helium,
neon, argon, krypton and xenon were discovered. With
the exception of helium, which is obtained from natural gas, all
the other members of this group are obtained from liquid air.
LIONEL
CHEM-LAB 55
There is very little helium in the atmosphere,
only about four parts in every million. Next to hydrogen it is the
lightest of all gases, but still is about twice as heavy as
hydrogen. The United States is able to produce more helium than
any other country because of our natural gas wells in Texas and
Utah. It has thus supplanted hydrogen in U. S. Navy’s
lighter-than-air craft for some years. It does not have quite as
much lifting power as hydrogen, but its safety factor more than
outweighs this small disadvantage - it being non-inflammable.
Of the other rare gases in the helium group,
neon and argon are the only ones having any commercial importance.
We first applied neon gas in a commercial way, about fifteen years
ago when neon signs came into existence. These glass tube signs
contain a very small amount of neon gas and when a current of
electricity is passed through the tube, the characteristic red
glow of neon appears. Recent developments in the field of electric
signs have now made it possible to improve greatly on the original
design of neon signs so that a great variety of colors and effects
are obtainable today, many without the use of neon.
REDUCTION
We have already seen that the name given to
the process when oxygen combines with an element to form an oxide,
is oxidation.
The reverse of this process can also take
place, that is, the oxide can give up its oxygen and the name
given to the process is reduction. Various substances such
as sodium bisulfite or hydrogen may be used as reducing agents or
we can use our blowpipe to accomplish this purpose.
EXPERIMENT
No. 82 The Reduction Of An Iron Compound
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, ferric ammonium sulfate, magnet, blowpipe,
charcoal block, candle.
PROCEDURE:
Place on your charcoal block a mixture of one measure of sodium
carbonate and a half measure of ferric ammonium sulfate.
Direct a reducing flame at this mixture by means of the
blowpipe. Heat it for two or three minutes (see instructions
on use of the blow-pipe). Pass a magnet over the tiny pieces and
observe what happens.
SUMMARY:
When the flame is directed at this mixture, we are able to reduce
the ferric or iron compound to its metallic state, proved by the
fact that it is attracted by the magnet.
EXPERIMENT No. 83 Reduction Of Iron Salicylate
(CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, sodium salicylate, test tube, sodium
bisulfite.
56 THE
ATMOSPHERE
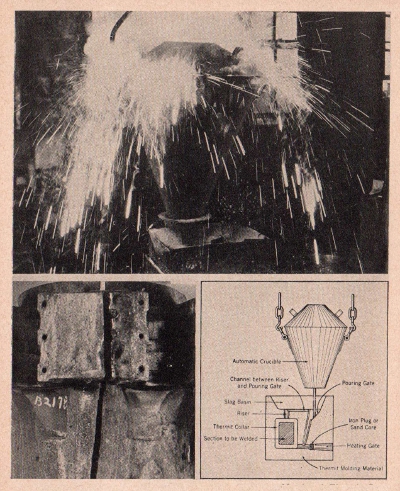
Metal and Thermit Corp.
The upper photograph shows the heat reaction resulting
from the ignition of aluminum and iron oxide used in Thermit welding.
Lower left is an example of a typical casting fracture requiring
the thermit weld. Lower right is a diagram of the actual welding
process wherein the molten metal is poured into the form built
around the fractured casting.
LIONEL
CHEM-LAB 57
PROCEDURE:
Put one eighth of a measure of ferric ammonium sulfate and an equal
amount of sodium salicylate in a test tube and add water up to the
three quarter mark. Note the color of the solution. Add five
measures of sodium bisulfite and shake well. Again note whether any
change in color occurs.
SUMMARY:
The deep red solution is ferric salicylate. Sodium bisulfite acts as
a reducing agent and readily reduces the ferric salicylate to
ferrous salicylate, a colorless compound. The reducing action takes
place on the iron which changes from a ferric to a ferrous state.
EXPERIMENT No. 84 Reduction Of Logwood
(CL-55, CL-66, CL-77)
APPARATUS:
Logwood, test tube, candle or alcohol lamp, sodium bisulfite.
PROCEDURE:
Fill a test tube three quarters full of water. Add two measures of
logwood and heat gently. Stop heating when the solution becomes a
deep red and pour this off into another test tube. Add five measures
of sodium bisulfite. Shake well and note any changes.
SUMMARY:
Sodium bisulfite again acts as a reducing agent and removes oxygen
from the logwood. When this takes place, the solution becomes
colorless.
THERMIT
Thermit welding has often been called
industry’s master maintenance tool as this form of welding makes
it possible to repair broken parts on heavy machinery,
locomotives, ships’ rudder frames, anchors and other large
castings which, due to their large size, otherwise could not be
repaired.
The thermit reaction is essentially a reduction
of iron oxide with aluminum used as the reducing agent. Thermit
itself is a mechanical mixture of finely divided aluminum and iron
oxide. When this mixture is ignited, a reaction of tremendous heat
occurs. The aluminum, having a high affinity for oxygen, leaves
the iron in a pure metallic form which, because of its weight,
falls to the bottom of the mixture. The molten iron is then
directed into a form built around the portion to be welded so that
the area surrounding the broken part itself is heated to a very
high temperature. The whole mass then integrates or fuses and the
seam or crack is entirely obliterated. When the casting is cool,
the form is removed, the surplus material cleaned off and the
casting is as good as new.
In times of war, thermit incendiary bombs are
widely used. The tremendous heat generated by these bombs makes it
exceedingly difficult to combat them. Dry sand is considered the
most effective extinguisher to use.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook