The
Science Notebook
Lionel Chem-Lab
- Chapter 4
The
Science Notebook
Lionel Chem-Lab
- Chapter 4
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 58 - 78
58 THE
STORY OF WATER
The three familiar states of water--liquid, gas, and
solid. Upper left is an example of the liquid state. Upper
right shows water in a gaseous state, Lower left, and the
microphotograph of the snowflake made by the General Electric
Laboratories, show water in a solid state.
CHAPTER IV
The Story of Water
Water is as essential to life as air and of all
the many chemical compounds available to man, it is certainly the
most common, the most abundant and the most useful. It is a
compound of two gases, hydrogen and oxygen, and has the well-known
formula H20. It covers approximately three-fourths of
the earth’s surface, mostly in the form of our great seas and
oceans, some of which extend to depths of over five miles.
Both plants and animals consist of from 60% to
95% water. The human body itself is approximately 70% water.
In our study of the air and atmosphere, we
observed that the air contains a large amount of water in the form
of vapor. In, this form, water controls weather conditions. Rain,
snow and other atmospheric precipitation regulate the productivity
of the soil, the habits and behavior of people, and in fact,
influence to a large extent the progress of civilization. This is
how important water is to us.
PROPERTIES OF WATER
Absolutely pure water has neither color, taste
nor odor. Probably you have noticed that the water in oceans,
lakes and rivers sometimes has a blue or green color. This is due
chiefly to the effect of sunlight, the presence of micro-organisms
and to mineral and other substances.
EXPERIMENT No. 85 Color And Clarity Of Water
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Test tube and paper.
PROCEDURE:
Hold a test tube filled with faucet water against a large sheet of
white paper. Note whether the water is clear and colorless.
SUMMARY:
Water in small quantities is colorless unless impurities are
present.
EXPERIMENT No. 86 Testing Water For Odor
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Test tube, candle or alcohol lamp.
59
60 THE
STORY OF WATER
PROCEDURE:
Fill a test tube about half full of water. Shake well, noting any
odor which may be present. Heat carefully and smell the water again.
SUMMARY:
In most cases, no odor will be present in the water either before or
after heating although sometimes heat will liberate gaseous
compounds dissolved in the water. Under these circumstances, an odor
may be detectable.
DEGREES OF PURITY
Water which falls as rain, snow and other
precipitation is the purest form of water found in nature, but is
not entirely pure, due
to the presence of dust particles and small traces of mineral and
organic matter. In the following experiments only in certain cases
will definite reactions be noticed because of the high degree of
purity found in most drinking water.
EXPERIMENT No. 87 Testing Water For Solid Matter
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Beaker or saucer, candle or alcohol lamp.
PROCEDURE:
Fill a beaker about half full of water. Heat gently until the water
evaporates. Examine the dry beaker and note whether any residue is
left.
SUMMARY:
If a thin residual coating is left after the water evaporates, it is
due to solid matter dissolved in the water.
EXPERIMENT No. 88 Testing Water For Acidity
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Blue litmus paper and test tube.
PROCEDURE:
Fill a test tube half full of water. Drop in a strip of blue litmus
paper. Note whether a change in color occurs.
SUMMARY:
Water in its natural state often dissolves decayed organic
substances. In most cases, no color reaction in the litmus paper
occurs. If the blue litmus paper turns red, it is due to traces of
acid in the water.
EXPERIMENT No. 89 Testing Water For Alkalinity
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Test tube and phenolphthalein solution.
PROCEDURE:
Add a few drops of phenolphthalein solution to a test tube half full
of water. Note whether there is any change in color.
SUMMARY:
The indicator, phenolphthalein, turns red in the presence of bases.
EXPERIMENT No. 90 Sulfates In Water
(CL-33, CL-44, CL-55, CL-66, CL-77)
LIONEL
CHEM-LAB 61
APPARATUS:
Strontium chloride and test tube.
PROCEDURE:
Dissolve one measure of strontium chloride in a test tube half full
of water. Shake well and allow to stand for a few minutes. Note
whether or not a precipitate appears.
SUMMARY:
If a sulfate is present, the solution becomes turbid and a
precipitate, strontium sulfate, will appear.
EXPERIMENT No. 91 Iron In Water
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide and test tube.
PROCEDURE:
Dissolve one measure of sodium ferrocyanide in test tube half full
of water. Shake well and allow to stand for awhile. Observe
any color changes.
SUMMARY:
If iron is present in water, a blue color appears when sodium
ferrocyanide is added.
EXPERIMENT No. 92 Hydrogen Sulfide In Water
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfide test paper and test tube.
PROCEDURE:
Fill a test tube half full of water. Insert a strip of sulfide test
paper. Note any color changes on the paper.
SUMMARY:
When moistened sulfide test paper (lead acetate) is brought into
contact with hydrogen sulfide, the paper becomes blackened because
of the formation of lead sulfide. This is a test for hydrogen
sulfide.
EXPERIMENT No. 93 Testing Water For Carbon
Dioxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide and two test tubes.
PROCEDURE:
Place one measure of calcium oxide in a test tube half filled with
water. Shake this solution of lime water well and allow the
undissolved particles to settle out. Pour a few drops of the clear
solution into a test tube half full of water. Note whether or not
any changes occur.
SUMMARY:
If water contains carbon dioxide gas or a carbonate, the addition of
limewater will bring about a cloudy and turbid solution. To get a
more positive reaction, repeat experiment using charged (soda)
water.
In the laboratory, the purest form of water is
obtained by distillation.
Distilled water is used in automobile storage batteries and in
chemical laboratories because mineral salts normally found in
water interfere with the exactness required of certain chemical
analyses.
Water can be distilled very easily by setting
up a simple laboratory
62 THE
STORY OF WATER
apparatus. The water to be distilled is placed
in a test tube and heated. The vapors are passed through the
delivery tube and condensed into a test tube immersed in water.
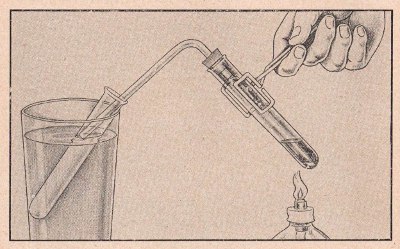
EXPERIMENT No. 94 Distillation Of Water
(CL-55, CL-66, CL-77)
FIGURE 11
APPARATUS:
Copper sulfate, gas delivery tube and stopper, two test tubes,
glass, alcohol lamp or candle.
PROCEDURE:
Place one measure of copper sulfate into a test tube half filled
with water. Shake to dissolve and note the blue color. Insert the
gas delivery tube and stopper into the test tube and place the other
end of the delivery tube in an empty test tube immersed in a glass
of cold water. Hold the test tube containing the copper sulfate
solution over a flame and heat to boiling. Note the condensation of
clear water in the other test tube.
SUMMARY:
Water turns to steam when it is heated, passes through the delivery
tube and condenses as water again when it reaches the cool test
tube. Copper sulfate has a higher boiling point than water,
therefore it remains behind in the first test tube.
Neither boiling nor filtration results in pure
water although by first boiling the water, then filtering to
remove the suspended matter, it will be improved.
LIONEL
CHEM-LAB 63
PURIFICATION OF DRINKING WATER
In addition to mineral impurities, we also
have to contend with the problem of organic matter found in
natural waters which may contain disease-producing organisms known
as bacteria.
There is a great danger of contamination to the
water supplies of our large cities because of the increasing
population and the fact that the water is usually obtained from
nearby lakes and rivers.
In order to prevent the spread of such a
disease as typhoid fever by drinking water, modern cities now
purify their entire water supply. We have already discussed
purification of water by distillation and boiling, but it is
obvious that such laboratory methods would not be practical on a
large scale. For this reason, the chlorine method of treatment,
sand filtration and aeration are the methods most widely used
either individually or jointly. Chlorination of drinking water is
discussed more fully in our chapter on Chlorine.
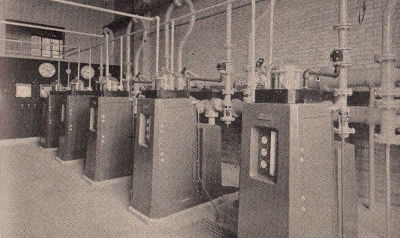
Wallace and Tiernan Co., Inc.
An illustration
of a modern chlorination plant showing the instruments used to
purify urban drinking water supplies and for decontaminating
sewage.
EXPERIMENT No. 95 Sand Filtration
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Fine-mesh tea strainer, sand and a glass.
64 THE
STORY OF WATER
PROCEDURE:
Fill the strainer with fine sand and place the strainer in the mouth
of the glass. Prepare some muddy water and pour it over the sand.
Note the color of the water as it drops into the glass.
SUMMARY:
The mud which remains in suspension in the water is removed as the
liquid filters through the sand. The filtrate will be quite clear,
any discoloration being due to foreign matter which cannot be
removed by the sand filter.
EXPERIMENT No. 96 Another Method Of Purifying
Water
(CL-66, CL-77)
APPARATUS:
Aluminum sulfate, calcium hydroxide, test tube.
PROCEDURE:
Dissolve a half measure of aluminum sulfate in a test tube three
quarters full of muddy water. Add one-half measure of calcium
hydroxide. Shake well and set aside for a few minutes. Note the
clarity of the water.
SUMMARY:
The suspended matter in the water can be gathered into larger
particles by introducing a little of the jelly-like precipitate of
aluminum hydroxide formed by the reaction of aluminum sulfate and
calcium hydroxide. The aluminum hydroxide settles out along with the
particles and thus the water becomes clear by the process of
coagulation.
HARD WATER AND SOFT WATER
We have already seen that water contains more
or less mineral and organic matter which it receives from rocks
and soil. This mineral material, composed principally of calcium
and magnesium, causes much trouble and expense. Soap does not
lather well in water having a high mineral content; there is a
waste of soap and washing becomes difficult. Also these minerals
settle out in the hottest parts of boilers and pipes forming a
stone-like coating which prevents full transfer of heat from the
fire. This is not only a nuisance and expensive to remove, but
sometimes results in boiler explosions.
EXPERIMENT No. 97 Testing Water For Hardness
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Soap shavings and two test tubes.
PROCEDURE:
Dissolve a small piece of soap in a test tube half full of warm
water. Add a few drops of this soap solution to a second test tube
full of water. Shake well and note whether there is any lather.
SUMMARY:
If the water lathers rapidly, the water is soft but if suds form
very slowly and a precipitate appears, the water is hard.
EXPERIMENT No. 98 The Reaction Of Soap With Hard
Water
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium chloride and two test tubes.
LIONEL
CHEM-LAB 65
PROCEDURE:
Dissolve one half measure of calcium chloride in a test tube half
full of water. Prepare a soap solution by dissolving a small piece
of soap in a second test tube half filled with warm water. Add
a few drops of the soap solution to the first test tube which now
contains hard water. Note the change in the solution.
SUMMARY:
The soap solution curdles in the presence of water made hard by the
addition of calcium chloride. Hard water is not suitable for washing
in the household or laundry because too much soap has to be
dissolved before the right amount of lather is obtained. Until the
salts which cause the hardness are precipitated, the water is not
suitable for washing.
Chemists differentiate between temporary hardness and permanent hardness.
Temporary hard water is frequently found in the
spring water of limestone regions. This water contains such
impurities as calcium bicarbonate and magnesium bicarbonate.
Temporary hard water can be softened by boiling, but on a large
scale this is not practical so a certain amount of calcium
hydroxide is used as a catalyst to precipitate insoluble calcium
carbonate. The purpose (as in boiling) is to convert the
soluble bicarbonate to insoluble carbonates.
Permanent hard water usually contains calcium
sulfate or chloride in solution. Sometimes also magnesium and iron
salts are present. The reason it is called "permanent" is because
it cannot be softened by boiling. When washing soda is added,
however, some of the impurities are precipitated.
Softening water on a large scale can be done
very efficiently by the Permutite
Process. Permutite is a complex substance composed
principally of sodium, silicon and aluminum.
ELECTROLYSIS OF WATER
We have mentioned that water is composed of
hydrogen and oxygen in the proportion of two parts of hydrogen to
one of oxygen. This can be proved by a process known as electrolysis, that is,
passing an electric current through the water.
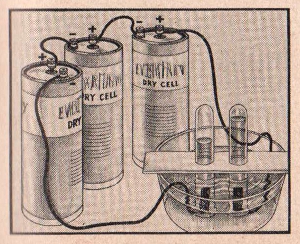
FIGURE 12
EXPERIMENT No. 99 Electrolysis Of Water
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Three dry cells, two test tubes, cardboard, a pan or any small
household utensil, copper wire.
66 THE
STORY OF WATER
PROCEDURE:
Connect the cells in series using insulated copper wire as shown in
the diagram. Wind the remaining ends of wire around the carbon rods.
Half fill the pan with water. Fill a test tube with water, place
your finger over the top and invert it in the pan removing your
finger when the mouth of the tube is in the water. Repeat this
procedure with the other test tube. Insert the carbon rods into the
two test tubes. Place ten measures of sodium bisulfate in the pan to
aid the passage of an electric current through the water. Hold the
test tubes erect by using a strip of cardboard as a brace. Note the
rising gas bubbles from the carbon posts, hydrogen gas from the
negative terminal, and oxygen from the positive terminal. After a
quantity of hydrogen and oxygen has been collected, remove the
carbon rods while keeping the test tubes under water. Note the
volume of gas in both tubes. Remove the tube containing the oxygen
and quickly insert a glowing splint. Note how the splint becomes
brighter (it may even burst into flame). Remove the tube containing
the hydrogen and cautiously place the mouth of the tube near the
flame. Note the slight
explosion.
SUMMARY:
Decomposition of a compound by the use of electrical energy is
called electrolysis.
After the apparatus for the experiment has
been set up in accordance with the diagram, the electric current
passing through the water acts to separate it into the two gases
of which it is formed. When water and other substances in solution
are decomposed by electric current the chemists explain this
action by the theory of ionization.
SOLUTIONS
In many of our experiments, we have made
solutions. What is a solution?
A solution is the result of dissolving one
substance (either solid, liquid or gas) in another substance,
usually a liquid. Although a solution is of uniform consistency,
the proportion of the ingredients may vary greatly and for this
reason we classify solutions as either dilute (having a small amount of dissolved
substance) or supersaturated.
EXPERIMENT No. 100 A Supersaturated Solution
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium thiosulfate, piece of cotton, test tube, candle or alcohol
lamp.
PROCEDURE:
Fill a test tube about one-third full of sodium thiosulfate. Add a
few drops of water and heat. When the solution is about to boil, set
it aside to cool, plugging the mouth of the tube with cotton.
Do not stir or shake while cooling. Remove the cotton and add a
small crystal of sodium thiosulfate and note the change.
LIONEL
CHEM-LAB 67
SUMMARY:
A supersaturated solution is one in which the quantity of the
substance dissolved is more than what the liquid can hold at
ordinary temperatures. Such a solution is very unstable as evidenced
by the way crystals of sodium thiosulfate begin to form when just
one additional crystal is dropped into the tube. The same effect can
be obtained by shaking or stirring.
EXPERIMENT No. 101 How A Solution Aids Chemical
Reactions
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bicarbonate (or sodium carbonate), tartaric acid, paper and
test tube.
PROCEDURE:
Mix on a sheet of paper one measure of tartaric acid and one measure
of sodium bicarbonate. Note whether any chemical reaction occurs.
Place this mixture in a test tube and add a few drops of water.
Observe whether a chemical reaction takes place.
SUMMARY:
When the chemicals were mixed no reaction took place, but the moment
a little water was added, a sizzling reaction set in and gas was
evolved. Water was a necessary factor in bringing about a chemical
reaction.
EXPERIMENT No. 102 Detecting Moisture
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, sodium ferrocyanide, white paper, test tube
and cotton.
PROCEDURE:
Dissolve three measures of ferric ammonium sulfate in a test tube
half filled with water. Immerse some strips of white paper in the
solution and allow to dry. Crush a crystal of sodium ferrocyanide
and apply it with a bit of cotton to one of the dry strips. Place a
drop of water in one corner of this strip and note the blue color.
SUMMARY:
When water is added to. a mixture of ferric ammonium sulfate and
sodium ferrocyanide, a blue color results. This is often used as a
test to detect the presence of water in a compound.
EXPERIMENT No. 103 Removing Salt From A Solution
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Table salt, glass and pan.
PROCEDURE:
Dissolve one heaping spoonful of salt in a glass one-quarter full of
warm water. Pour the contents into a pan and heat carefully on the
stove until the water evaporates. Note whether a solid remains. Test
the recovered salt by tasting.
SUMMARY:
The salt taste proves that the salt has been recovered. This
is not a difficult task because the water can be vaporized while the
salt cannot. This also proves that a substance does not necessarily
lose its properties by being dissolved in water.
68 THE
STORY OF WATER
EXPERIMENT No. 104 A Solvent For Grease
(CL-66, CL-77)
APPARATUS:
Carbon tetrachloride and butter.
PROCEDURE:
Insert a small portion of butter into a dry test tube. Add a
little carbon tetrachloride. Note that the butter dissolves.
SUMMARY:
Carbon tetrachloride is a good solvent for fats, oils, gums and
resins and thus is used extensively as a cleaning fluid.
EXPERIMENT No. 105 Temperature Affects
Solubility Of Gas
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Test tube and candle or alcohol lamp.
PROCEDURE:
Fill a test tube half full of water. Hold the test tube over a flame
and note the appearance of gas bubbles. Continue heating until the
water boils, then cool by running cold water on the test tube. Heat
again and note whether gas bubbles form. Repeat this process several
times.
SUMMARY:
In the first few heatings the gaseous compounds are removed from the
water, proving that the solubility of gas diminishes with an
increase in temperature. However, the lower the temperature of the
liquid, the greater the solubility of the gas. For a more positive
reaction, repeat the experiment substituting charged (soda) water.
OTHER TYPES OF SOLUTIONS
EXPERIMENT No. 106 Boiling A Solution Removes
Gas
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium hydroxide (or household ammonia), phenolphthalein solution,
test tube, candle or alcohol lamp.
PROCEDURE:
Place a drop of ammonium hydroxide in a test tube one-quarter full
of water. Add a drop of phenolphthalein solution and note the color
change. Heat carefully until the liquid boils and again note the
color change.
SUMMARY:
Ammonium hydroxide in water gives a basic reaction.
Consequently, it becomes red when phenolphthalein is added. When the
ammonia is heated, the ammonia gas is driven off with the result
that the solution loses its alkalinity and becomes colorless.
EXPERIMENT No. 107 Liquids Soluble In Each Other
(CL-66, CL-77)
APPARATUS:
Carbon tetrachloride, glycerine and two test tubes.
PROCEDURE:
Add a few drops of carbon tetrachloride to a test tube containing a
little water. Shake vigorously and note whether the carbon
tetrachloride dissolves. Place a few drops of glycerine in the other
LIONEL
CHEM-LAB 69
test tube containing water. Shake well and note whether the
glycerine dissolves.
SUMMARY:
There are times when one liquid will not dissolve in another as is
the case with water and carbon tetrachloride. Whenever this occurs,
the solvents settle out in individual layers. Glycerine, however, is
soluble in water.
FILTERING SUSPENSIONS
We have mentioned that a solution is a uniform
mixture, but a suspension is also a mixture of a solid and a
liquid. In the case of a suspension, however, the solid particles
settle out on standing. When finely powdered sand or clay
(insoluble in water) is well shaken or stirred in water, a turbid
liquid is obtained. This is an example of a suspension. The solid
sand does not dissolve and after the mixture has stood for a while
the sand will slowly settle to the bottom leaving the clear
liquid. This solid can be removed by the process of filtration.
HOW TO FILTER
To filter a solution you must use your glass
funnel and filter paper. Filter paper comes in round sheets and it
must be folded so that it will fit closely into the glass funnel.
Do this by folding a sheet of filter paper through the center and
then folding it again into quarters. After this has been done, it
can be made into a cone and set into the top of the funnel. You
are now ready to pour the solution into the funnel.
EXPERIMENT No. 108 Separating A Soluble From An
Insoluble
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, sulfur, test tube and filter paper.
PROCEDURE:
Mix five measures of ammonium chloride and two and a half measures
of sulfur in a test tube. Add water up to the three-quarter mark and
shake. Pass this through a filter and collect the filtrate in a
saucer. Heat the solution slowly to evaporate it. Unroll the filter
paper and allow contents to dry. Check these against the original
chemicals.
SUMMARY:
The ammonium chloride was soluble in the water whereas the sulfur
was not. Thus, the ammonium chloride passed through the filter and
was collected in solution as a filtrate. When this is heated, the
water is drawn off and the ammonium chloride remains as a solid in
the original state. By drying the filter paper you will be able to
recover the sulfur as it was originally.
TEMPERATURE
We have already mentioned that the freezing point of water is 32° F.
(Fahrenheit) and the boiling point is 212° F.
70 THE
STORY OF WATER
The chemist measures the temperature of a
substance with a thermometer, usually marked off in the degrees of
the Centigrade scale.
According to the Centigrade scale, water freezes at 0° C. and
boils at 100° C. However, as most boys are more familiar
with the Fahrenheit
scale, the Lionel Chem-Lab thermometer is graduated according to
this scale from 20° below zero to 230° above zero, or 18° above
the boiling point of water. This thermometer is suitable for
taking temperature readings in any of our experiments.
If we make up a solution, by dissolving some substance such as
salt in water, this very definitely affects the freezing and
boiling points. Likewise, it seems that solids dissolve more
readily in a warm liquid than they do at a cooler temperature.
Sugar, for example, is more soluble in hot water than in cold.
Salt, on the other hand, will dissolve nearly as well in cold as
in hot water.
Certain important changes take place while a
solution is being formed. When some solids are dissolved,
energy is absorbed and the temperature of the solution goes down.
For example, in preparing a freezing mixture, when we mix ice and
salt, some of the ice melts and the salt dissolves in the
water. Both of these processes result in the absorption of
heat, and thus the temperature will fall below the normal freezing
point of water. The salt water does not freeze because its
freezing point is lower than that of pure water.
In the old fashioned method of making ice
cream, the cream was placed in a metal container around which was
packed a mixture of salt and ice. The faster ice melts, the
greater the degree of coldness. Therefore, to accelerate the
freezing of the cream, the salt was used to melt the ice more
quickly.
EXPERIMENT No. 109 Comparing Boiling Points
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Salt, two test tubes, candle or alcohol lamp.
PROCEDURE:
Prepare a salt solution by dissolving fifteen measures of salt in a
test tube three-quarters full of water. Fill a second test tube
three-quarters full of water. Heat both test tubes until the
contents boil. Note that one solution boils sooner than the other.
SUMMARY:
When salt is added to water, the boiling is hastened.
EXPERIMENT No. 110 Comparing Freezing Points
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cracked ice, glass, two test tubes and salt.
PROCEDURE:
Prepare a freezing mixture by adding one third of a glass of salt to
a glass filled with cracked ice. Dissolve fifteen measures of salt
in a test tube half full of water. Fill a second test tube half full
of water. Place both test tubes in the freezing mixture. Note that
the plain water will freeze before the salt solution.
LIONEL
CHEM-LAB 71
SUMMARY:
When salt is added to water, its freezing point is lowered.
EXPERIMENT N0. 111 Supercooled Water
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, {CL-77)
APPARATUS:
Glass, cracked ice, salt, test tube, thermometer.
PROCEDURE:
Prepare a freezing mixture by adding one quarter of a glass of salt
to a glass three fourths full of cracked ice. Fill a test tube one
quarter full of water. Carefully insert the tube into the freezing
mixture. Check temperature after a short time. Now drop a piece of
ice into the test tube. Again check the temperature at which
freezing occurs.
SUMMARY:
A Supercooled solution is one which has been cooled below its
freezing point without becoming solid. This occurs only in a
solution which has not been disturbed during the cooling process.
However, the solution will freeze immediately if the test tube is
moved or a piece of ice added.
EXPERIMENT No. 112 Another Effect Of Temperature
On Solubility
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, two test tubes, candle or alcohol lamp.
PROCEDURE:
Add one measure of calcium oxide to a test tube half filled with
water. Shake vigorously and then allow the undissolved particles to
settle out. Carefully pour the clear solution into another test tube
and heat over a flame. Note change in the appearance of the clear
solution.
SUMMARY:
Calcium oxide is one of the few chemicals which are more soluble in
cold water than in hot. Consequently, some of the particles of
calcium oxide come out of the solution as it is heated and cause the
liquid to become cloudy.
EXPERIMENT No. 113 Negative Heat Of Solution
(CL-55, CL-66, CL-77)
APPARATUS:
Test tube, ammonium chloride (or sodium thiosulfate) and
thermometer.
PROCEDURE:
Take the temperature of a test tube half filled with water. Add one
half spoonful of ammonium chloride. Note that the temperature falls.
SUMMARY:
Some compounds such as ammonium chloride have the ability to absorb
heat from the water in which they are dissolved, consequently
cooling the water. Compounds of this type are said to have a
negative heat of solution.
EXPERIMENT No. 114 Positive Heat Of Solution
(CL-55, CL-66, CL-77)
72 THE
STORY OF WATER
APPARATUS:
Test tube, magnesium sulfate or calcium chloride, thermometer.
PROCEDURE:
Take the temperature of a test tube half full of water. Add a
half spoonful of magnesium sulfate. Note the rise in temperature.
SUMMARY:
Some compounds such as magnesium sulfate give off heat to the water
in which they are dissolved. Compounds of this type have a
positive heat of solution.
SPECIFIC GRAVITY
The term specific
gravity sounds formidable but it merely means that one
substance is more dense than another. Lead is more dense than
water; lead weighs more than water. Therefore, density is the
weight of a substance in a certain unit of volume.
EXPERIMENT No. 115 Specific Gravity
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium salicylate, ferric ammonium sulfate, piece of tissue paper
and a drinking glass.
PROCEDURE:
Dissolve two measures of sodium salicylate in a glass three fourths
full of water. Place one measure of ferric ammonium sulfate in the
tissue paper and fold the corners so that the chemical is completely
covered by the paper. Insert this in the glass containing the sodium
salicylate and set aside. Note the streamer formation and the
sinking of the red liquid.
SUMMARY:
When the sodium salicylate solution passes through the tissue paper
it reacts with the ferric ammonium sulfate to form the beautiful red
streamers of ferric salicylate. These streamers sink to the bottom
because the specific gravity has been changed.
The gram is the unit of weight generally used
when dealing with solids and liquids, and the cubic centimeter (cc.) is the
unit of volume. Specific gravity is defined, therefore, as
the weight in grams of 1 cc. of the substance. Since 1 cc.
of water weighs 1 gram, it can be seen readily that the number
which represents the density of other solids and liquids also
represents their relative
weights as compared with water.
SURFACE TENSION
EXPERIMENT No. 116 Surface Tension
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Rubber band, dish, toothpick and oil.
PROCEDURE:
Float a thin rubber band in a dish of water, making certain that it
does not touch the sides. By means of a toothpick dipped in oil,
pierce the surface of the water inside the rubber band. Do it
LIONEL
CHEM-LAB 73
again and note the results. Repeat, this time piercing the water
outside of the band. Again note the results.
SUMMARY:
When oil is applied to the water area within the rubber band, the
surface tension of this area becomes less than the surface of the
water surrounding the rubber band. Thus the band is stretched away
from its center. When the oil is applied in the same fashion to the
liquid outside the band, the surface tension outside becomes less
than the surface tension inside and the rubber band returns to its
original shape.
EXPERIMENT N0. 117 A Needle Floats On Water
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Saucer and sewing needle.
PROCEDURE:
Carefully place a small sewing needle on the surface of the water in
a saucer. Note whether the needle has a tendency to sink.
SUMMARY:
The needle floats because the surface film, which has not been
pierced or distorted, supports it.
EXPERIMENT No. 118 Destroying Surface Tension
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Saucer, sodium carbonate, glass, a needle, soap.
PROCEDURE:
Dissolve several measures of sodium carbonate in a saucer half full
of water. Carefully place the needle on the surface of the solution
and touch the water on each side of the needle with soap. Observe
whether the needle sinks or floats.
SUMMARY:
Soap in this particular case destroyed the surface film allowing the
needle to sink.
EXPERIMENT No. 119 Rise Of Water In Capillary
Tubes
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Glass tube, candle or alcohol lamp, dish and file.
PROCEDURE:
Heat the middle section of the glass tube over the flame rolling it
back and forth between your fingers. Remove from the flame when
glass becomes soft and pull carefully in opposite directions to
decrease the diameter of the central portion of the tube. Cut this
thin section out with a file. Insert it in the bowl of water and
note the rise of the solution in the tube.
SUMMARY:
The water in the tube rises above the surface of the water in the
dish. This demonstrates capillary action.
EXPERIMENT No. 120 Capillary Action In A Lump Of
Sugar
(CL-66, CL-77)
APPARATUS:
Mixed dyes, teaspoon, lump of sugar and saucer.
74 THE
STORY OF WATER
PROCEDURE:
Add one half measure of mixed dyes to a saucer containing a few
teaspoonfuls of water. When a uniform color exists, stand a lump of
sugar upright in the liquid. Note the rise of color in the lump of
sugar.
SUMMARY:
The colored solution aids in detecting the rise of water. This
is a form of capillary action because the spaces between the grains
of sugar act as capillary tubes.
DIFFUSION
Diffusion
means the gradual dispersion of the particles of one substance
among those of another. The four principal types of diffusion are:
diffusion of liquids, diffusion of substances in solution,
diffusion of gases and diffusion of solids.
EXPERIMENT No. 121 Diffusion Demonstrated
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, tall glass and cobalt chloride, test tube.
PROCEDURE:
Fill a test tube one-quarter full of sodium silicate solution. Add
water to the half way mark and stir thoroughly. Add three or four
crystals of cobalt chloride. Note the growth. Float a cobalt
chloride crystal on the surface of the liquid. Note the growth
toward the bottom of the glass.
SUMMARY:
The swelling of the cobalt chloride crystals is due to their
reacting with the sodium silicate solution to form cobalt silicate.
EXPERIMENT No. 122 Color Uniformity In A
Solution
(CL-66, CL-77)
APPARATUS:
Mixed dyes and glass.
PROCEDURE:
Add a very small amount of mixed dyes to a glass half filled with
water. Set aside and note whether a uniform color finally sets in.
SUMMARY:
The dye diffuses through the liquid until the solution has a uniform
color. Ordinarily a solid when dissolved tends to distribute itself
uniformly through the liquid so that every part of the solution has
the same concentration. This goes on very slowly unless it is
speeded by stirring or shaking. In the case of some colored solids
dissolved in water, weeks may go by before a uniform color is
obtained.
EXPERIMENT No. 123 Diffusion Of Red Ferric
Salicylate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, sodium salicylate and test tube.
PROCEDURE:
Place a few crystals of ferric ammonium sulfate in a test tube two
thirds full of water. Carefully add one measure of
LIONEL
CHEM-LAB 75
sodium salicylate. Note the reaction. Set aside for a few days and
observe the uniformity of the red solution.
SUMMARY:
The red ferric salicylate goes to the bottom of the liquid because
of specific gravity. However, after standing for a long time, the
red portion diffuses through the liquid and results in a uniform
color.
WATER OF CRYSTALLIZATION
Many substances combine chemically with water
to form compounds called hydrates. These compounds will give up or
lose water by simple exposure to the air or application of heat.
Water occurring and behaving in compounds in this interesting way
is known as water of
crystallization or hydration.
EXPERIMENT No. 124 Changing The Color Of A
Solution
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, test tube, candle or alcohol lamp.
PROCEDURE:
Add three measures of cobalt chloride to a test tube half full of
water. Heat gently and note color change. Allow tube and contents to
cool and again observe the change in color.
SUMMARY:
Heating the solution drives off the water of hydration of cobalt
chloride turning it blue. However, as the solution cools, the cobalt
chloride again takes up the water which it has lost and becomes
pink.
Many compounds which are soluble in hot water
give a visible crystalline precipitate upon cooling. Crystalline
washing soda (sodium carbonate) is an example of a compound which
behaves in this way.
EXPERIMENT No. 125 Washing Soda Crystals
(CL-11, CL-22. CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Washing soda (sodium carbonate), test tube.
PROCEDURE:
Place four measures of washing soda in a test tube. Heat
carefully. Note that the water condenses at the mouth of the test
tube.
SUMMARY:
Washing soda is sodium carbonate plus water of hydration. When heat
is applied, the water is driven off and the plain sodium carbonate,
which is white in appearance, remains.
Crystals vary in size from those which can
only be seen with the aid of a microscope to such massive types as
the quartz crystal in California which weighs a ton.
The secret of making better crystals is to
permit the solution to cool slowly. Keep this in mind when
performing subsequent experiments.
76 THE
STORY OF WATER
EXPERIMENT No. 126 Tartaric Acid Crystals
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tartaric acid, beaker or small glass, cardboard, alcohol lamp or
candle, test tube.
PROCEDURE:
Prepare a saturated solution of tartaric acid by dissolving as much
of the chemical as possible in a test tube filled with hot water.
Pour the clear liquid into a small glass or beaker. Cool slowly,
placing a small piece of cardboard over the mouth of the
glass. Examine crystals for structure and appearance.
EXPERIMENT No. 127 Magnesium Sulfate Crystals
(CL-66, CL-77)
APPARATUS:
Magnesium sulfate, alcohol lamp or candle, test tube, beaker or
small glass, piece of cardboard.
PROCEDURE:
Prepare a saturated solution of magnesium sulfate by dissolving as
much of the chemical as possible in a test tube filled with water.
Pour the clear liquid into a small glass or beaker. Cool slowly,
placing a cardboard over the mouth of the glass. Examine crystals
for structure and appearance.
EXPERIMENT No. 128 Sodium Sulfate Crystals
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium sulfate, test tube, beaker or small glass, piece of
cardboard, alcohol lamp or candle.
PROCEDURE:
Prepare a saturated solution of sodium sulfate by dissolving as much
of the chemical as possible in a test tube half full of hot
water. Pour the clear liquid into a small glass or
beaker. Cool slowly by placing a cardboard over the mouth of
the glass. Examine crystals for structure and appearance.
EXPERIMENT No. 129 Crystal Formation
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium borate, test tube, candle or alcohol lamp.
PROCEDURE:
Place ten measures of sodium borate in a test tube one quarter full
of water. Boil until the solid dissolves. Set contents aside to
cool. Repeat as above but this time cool the contents quickly by
inserting the test tube in a glass of water.
SUMMARY:
When the solution cools quickly, frost-like crystals begin to form
on the walls of the test tube. The slower the cooling process, the
better the crystals.
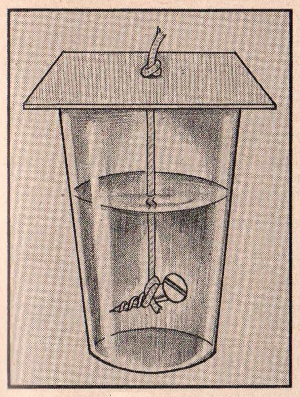
EXPERIMENT No. 130 Formation Of Rock Salt
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
LIONEL
CHEM-LAB 77
FIGURE 13
APPARATUS:
Small glass, common salt, cardboard, string, weight and stirring
rod.
PROCEDURE:
Fill a glass about one quarter full of salt. Add water until the
glass is three fourths full. Dissolve all the salt by stirring,
adding a little more water, if necessary. Suspend a string in
the glass by attaching one end to a piece of cardboard resting on
top of the glass, and fastening a small weight to the end going into
the solution. Cover the glass and set aside for a few days noting
any changes which occur from day to day.
SUMMARY:
Within s day or two salt crystals will begin to form on the string
and the size of these crystals will increase daily.
EXPERIMENT No. 131 How To Make Rock Candy
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Two glasses, string, small weight, cardboard, sugar and stirring
rod.
PROCEDURE:
Dissolve as much sugar as possible in a small glass half filled with
hot water. Stir thoroughly and pour the clear syrupy liquid into
another glass. Suspend a string in the glass as in the preceding
experiment. Set aside for a few days.
SUMMARY:
Within a few days crystals of sugar, commonly called rock candy,
will form on the string.
EXPERIMENT No. 132 Frosting Glass With Crystals
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, glue, test tube, small brush, pane of glass.
PROCEDURE:
Place six measures of ammonium chloride and one drop of glue in a
test tube containing one half inch of water. Boil until the solid
dissolves. Brush the hot liquid on the pane of glass. Note the
crystal formation.
SUMMARY:
The crystals will appear as soon as the heated liquid has been
applied to the glass making it appear as if it were frosted with
ice.
78 THE
STORY OF WATER
EXPERIMENT No. 133 Efflorescence Demonstrated
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, heating spoon, candle or alcohol lamp and stirring
rod.
PROCEDURE:
Observe the color of cobalt chloride when two measures of it are
placed in the heating spoon. Heat gently until it becomes
blue. Add a drop of water, stir and once again note the color.
SUMMARY:
Cobalt chloride loses its water, or dehydrates, when heated and
turns blue. This process is known as efflorescence. Adding a drop of
water causes the original pinkish color to return.
EXPERIMENT No. 134 A Cobalt Chloride Barometer
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, white paper, test tube and a small brush.
PROCEDURE:
Prepare a concentrated solution of cobalt chloride by dissolving two
measures in a test tube one quarter full of water. Brush this
solution on a piece of white paper and allow to dry. Hang the paper
in a place where the outdoor air can get at it easily.
SUMMARY:
Cobalt chloride is sensitive to moisture in the air. When it
acquires a bluish tinge, it usually indicates fair weather ahead,
and when it becomes pink, the indication is that dampness has set in
or rain is about to fall.
EXPERIMENT No. 135 Deliquescence Demonstrated
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium chloride, saucer or watch glass.
PROCEDURE: Place two measures of calcium chloride on a saucer.
Allow it to remain exposed to the air for some time. Examine
occasionally and note changes.
SUMMARY:
If the air is not too dry, calcium chloride will absorb moisture
from it and become wet. This is known as deliquescence.
Calcium chloride is frequently sprinkled on dusty roads and subway
tracks to keep the road moist and allow the dust to settle.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook