The
Science Notebook
Lionel Chem-Lab
- Chapter 7
The
Science Notebook
Lionel Chem-Lab
- Chapter 7
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 112 - 120
CHAPTER VII
SILICON AND THE SILICATES
Of the three most important elements in nature,
everyone agrees that oxygen and carbon, because of their
prevalence and importance, rank first and second. And a chemist
would quickly tell you that the third ranking is silicon because
of its importance in the solid structure of the earth. Silicon
itself makes up at least 25% of the earth’s substances while its
compounds comprise at least 87%.
Pure silicon is not found free in nature but
occurs chiefly in the form of silicon dioxide, otherwise known as
silica, or sand and sandstone. In crystalline forms, it exists as
amethyst, rose quartz, smoky quartz, onyx, opal, agate and flint.
EXPERIMENT No. 201 Sodium Silicate Or "Water
Glass"
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, paper, splints of wood, red litmus paper
and a small soft brush.
PROCEDURE:
Using a brush, paint some sodium silicate solution on a sheet of
paper. Allow paper to dry for fifteen minutes. Note the glassy film.
Place some silicate solution on a match stick and hold it against
another match stick. Let the sticks dry and then try to separate
them. Insert a piece of red litmus paper into the solution and note
the color change.
SUMMARY:
Sodium silicate is often used as an adhesive and to give a glazed
appearance to pottery. Sodium silicate turns red litmus paper blue
because it is made from a weak acid (silicic acid) and a strong base
(sodium hydroxide).
EXPERIMENT No. 202 Silicic Acid
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, sodium bisulfate and test tubes.
PROCEDURE:
Pour some sodium silicate solution in a test tube one quarter filled
with water and shake well. Dissolve three measures of sodium
bisulfate in a test tube one quarter filled with water. Add to this
the sodium silicate solution and note the jelly-like precipitate.
Allow to stand for a few minutes and note that the precipitate
solidifies. The jellied precipitate is silicic acid.
112
LIONEL
CHEM-LAB 113
EXPERIMENT No. 203 Silicon Dioxide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, sodium bisulfate, test tubes, stirring
rod, heating spoon and alcohol lamp.
PROCEDURE:
Pour a little sodium silicate solution in a test tube one-quarter
filled with water and shake test tube well. Dissolve three measures
of sodium bisulfate in another test tube one-quarter filled with
water. Add to this the sodium silicate solution and note the
jelly-like precipitate. Allow to stand for a while and observe that
the precipitate solidifies as silicic acid. Place some of this
precipitate in the measuring spoon and heat it over a flame for five
minutes. Note that the silicic acid precipitate dries into a white
solid.
SUMMARY:
When silicic acid is heated it loses water and becomes silicon
dioxide (sand).
Compounds consisting of silicon and one of the
elements are called silicides
of which the most important is carbon
silicate, or carborundum. After carbon and sand are
heated in an electric furnace and silicon is formed, under the
proper conditions silicon will combine with carbon to form
carborundum, an extremely hard substance, second only to the
diamond in its degree of hardness.
EXPERIMENT No. 204 Cobalt Silicate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, sodium silicate solution and test tube.
PROCEDURE:
Place two measures of cobalt chloride in a test tube half-filled
with water. Shake to dissolve. To this solution, add a few drops of
sodium silicate and note the blue precipitate.
SUMMARY:
The cobalt reacts with the silicate to form the blue precipitate.
EXPERIMENT No. 205 Strontium Silicate
(CL-33, CL-44, CL-55. CL-66, CL-77)
Repeat Experiment No. 204 substituting strontium chloride for cobalt
chloride. The precipitate will be strontium silicate.
EXPERIMENT No. 206 Manganese Silicate
(CL-33, CL-44, CL-55, CL-66, CL-77)
Repeat Experiment No. 204 substituting manganese sulfate for cobalt
chloride. The precipitate will be manganese silicate.
EXPERIMENT No. 207 Magnesium Silicate
(CL-66, CL-77)
Repeat Experiment No. 204 substituting magnesium sulfate for cobalt
chloride. The precipitate will be magnesium silicate.
114 SILICON
AND THE SILICATES
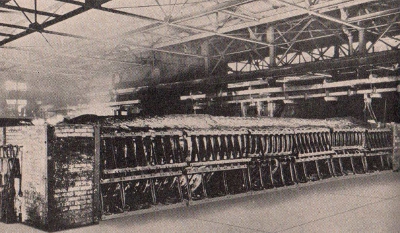
A
cross-section diagram of an electric furnace used in
manufacturing carborundum. The process consists of passing a
strong electric current through a mixture of sand, sawdust, coke
and salt.
Photograph
of an electric carborundum furnace used by the Carborundum
Company.
LIONEL
CHEM-LAB 115
EXPERIMENT No. 208 Ferric Silicate
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
Repeat Experiment No. 204 substituting ferric ammonium sulfate for
cobalt chloride. The precipitate will be ferric silicate.
EXPERIMENT No. 209 Ferrous Silicate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 204 substituting ferrous ammonium sulfate for
cobalt chloride. The precipitate will be ferrous silicate.
EXPERIMENT No. 210 Copper Silicate
(CL-55, CL-66, CL-77)
Repeat Experiment No. 204 substituting copper sulfate for cobalt
chloride. The precipitate will be copper silicate.
EXPERIMENT No. 211 Chromium Silicate
(CL-44, CL-55, CL-66, CL-77)
Repeat Experiment No. 204 substituting chrome alum for cobalt
chloride. The precipitate will be chromium silicate.
EXPERIMENT No. 212 Aluminum Silicate
(CL-33, CL-44, CL-55, CL-66, CL-77)
Repeat Experiment No. 204 substituting aluminum sulfate for cobalt
chloride. The precipitate will be aluminum silicate.
EXPERIMENT No. 213 Calcium Silicate
(CL-33, CL-44, CL-55, CL-66, CL-77)
Repeat Experiment No. 204 substituting calcium chloride for cobalt
chloride. The precipitate will be calcium silicate.
EXPERIMENT No. 214 Tin Silicate
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, ammonium chloride, tin metal (tin can), sodium
silicate solution and test tubes.
PROCEDURE:
Place a small piece of tin metal, two measures of sodium bisulfate
and two measures of ammonium chloride in a test tube. Add a few
drops of water and boil for a few minutes. Stop heating and pour off
the clear solution into another test tube to which more water is
added up to the one quarter mark. Add a few drops of sodium silicate
solution and note the thick white precipitate.
SUMMARY:
The dissolved tin reacts with the sodium silicate to form the thick,
milky precipitate of tin silicate.
EXPERIMENT No. 215 Zinc Silicate
(CL-44, CL-55, CL-66, CL-77)
116 SILICON
AND THE SILICATES
APPARATUS:
Zinc metal, sodium bisulfate, test tubes, alcohol lamp or candle,
sodium silicate solution.
PROCEDURE:
Put a small piece of zinc and four measures of sodium bisulfate in a
test tube. Add water up to the half-way mark. Heat until a little of
the zinc dissolves. Allow tube and contents to cool by immersing in
a glass of cold water. Add to the test tube a few drops of sodium
silicate solution and note the greyish-white precipitate.
SUMMARY:
The dissolved zinc reacts with the sodium silicate to form the
heavy, greyish-white precipitate of zinc silicate.
EXPERIMENT No. 216 Vegetation In A Drinking Glass
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, copper sulfate, ferric ammonium sulfate,
cobalt chloride, chrome alum, calcium chloride, manganese sulfate,
tumbler and stirring rod.
PROCEDURE:
Pour two spoonfuls of sodium silicate solution into a tumbler one
quarter filled with water and stir thoroughly. Add to this a few
crystals of ferric ammonium sulfate, cobalt chloride, chrome alum,
calcium chloride, copper sulfate and a pinch of manganese sulfate.
Allow the glass to remain undisturbed for a few hours and note the
growth.
SUMMARY:
The crystals grow like plants and make a beautiful sight with the
formation of many colors.
The silicate industries include those
manufacturing glass, cement, brick, tile, pottery and chinaware.
To make glass, a silicate mixture is prepared
by melting certain metal compounds with sand at an extreme
temperature. This mixture is then completely melted and cooled
without crystallization. Because of the tendency of glass to be
very soft and plastic when in the cooling state, it can be molded
or blown in the glass factory to practically any desired shape and
size. Your Chem-Lab bottle, for example, was molded. In the
molding process, glass is forced into a cavity corresponding in
shape and size to the desired article. After a little molten glass
has been introduced to the mold, compressed air is blown in which
forces the glass outward so that it assumes the form and shape of
the mold. The article is then cooled, the mold opened, and the
finished pieces removed. The excess material around the top of the
bottle is removed and the sharp edges rounded off in a flame. Most
bottles are made today by automatic machinery.
Window glass used to be made from cylinders of
glass blown by skilled glass blowers, but mechanical operations
have almost entirely superseded this method. The molten glass is
now rolled between hot rollers and large, flat sheets are thus
made. Plate glass, on the other hand, is cast into large flat
slabs which, after grinding and polishing, have perfectly plane
surfaces.
LIONEL
CHEM-LAB 117
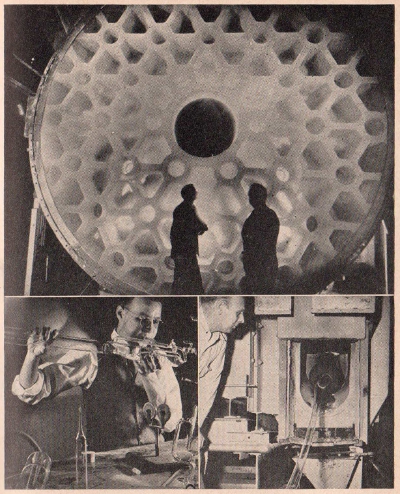
Top
photograph shows the great 200-inch mirror made by the Corning
Glass Works for the Mount Palomar Observatory. It is the largest
single piece of glass ever poured.
Lower
left shows a glass blower at work.
Lower right
shows silicate-glass tubing for fluorescent lamps pouring from a
General Electric Company furnace.
118 SILICON
AND THE SILICATES
EXPERIMENT N0. 217 Soft Glass
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, sodium carbonate, calcium oxide, alcohol
lamp, heating spoon, blowpipe.
PROCEDURE:
Pour five or six drops of sodium silicate solution in a heating
spoon. Add one measure of sodium carbonate and a half-measure of
calcium oxide. Mix the contents thoroughly. Direct the flame of the
alcohol lamp at this with the blowpipe, and note how it swells,
eventually becoming shiny and glassy.
SUMMARY:
Soft glass is commercially used for making bottles and drinking
glasses.
EXPERIMENT No. 218 Why Soft Glass Cracks Easily
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Glass tubing, alcohol lamp or candle, a glass.
PROCEDURE:
Heat a small piece of glass tubing. Immerse quickly in a glass of
water. Note that the tube cracks immediately.
SUMMARY:
Soft glass will crack if cooled quickly because the outside of the
glass is cooled more rapidly than the inside which sets up a strain.
EXPERIMENT No. 219 How To Make Hard Glass
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, sodium carbonate, calcium oxide, borax,
heating spoon, alcohol lamp, blowpipe.
PROCEDURE:
Mix a half measure of sodium carbonate, a half measure of calcium
hydroxide and one measure of borax with eight drops of sodium
silicate solution. Place the mixture on the heating spoon and heat
it by directing the flame of the alcohol lamp at the mixture with
the blowpipe. Note that the mixture froths for a while and
eventually melts into a clear, glassy bead.
SUMMARY:
Borax is one of the materials which can be added to glass enabling
it to withstand sudden changes in temperature without cracking.
EXPERIMENT No. 220 How To Frost Glass
(CL-66, CL-77)
APPARATUS:
Magnesium sulfate, liquid glue and a glass.
PROCEDURE:
Dissolve four teaspoonfuls of magnesium sulfate in a glass of water.
Add a teaspoonful of liquid glue and stir. Apply this to the object
to be frosted and set aside to dry.
SUMMARY:
Note the frosted appearance.
LIONEL
CHEM-LAB 119
EXPERIMENT No. 221 Etching Glass
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Glass, tumbler, strong liquid glue and small brush.
PROCEDURE:
Spread some heavy liquid glue on the outside and inside surfaces of
a glass tumbler. Set the tumbler aside to dry for a day and then
place it near a stove for another day. Move the tumbler to a cool
place and note that after awhile the glue begins to crack and chip
off. Allow it to remain in this cool spot for a short time and then
wash it with warm water to remove all the glue.
SUMMARY:
Note the etched surface caused by the cracking of the dried glue.
The liquid glue attacked the silicate in the glass and when the glue
was dried and began to chip off, some chips of glass came off with
the glue.
There are so many varieties and colors of
glass, you may wonder how they are made. Sometimes, merely by
adding a chemical, marvelous changes rake place. For example,
Pyrex glass which is used to make our Chem-Lab beakers and
Erlenmeyer flasks is able to stand sudden changes of temperature
and is not easily broken because sodium aluminum borosilicate was
added to the sand to make it especially resistant. Optical glass
gets its brilliancy because lead silicate is used. Colored glass
is made in dozens of shades by adding such chemicals as cobalt
compounds for blue, gold for ruby red, and manganese for purple.
EXPERIMENT No. 222 Making Light Green Glass
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, sodium carbonate, calcium oxide, ferrous
ammonium sulfate, alcohol lamp, heating spoon, blowpipe.
PROCEDURE:
Mix on a clean sheet of paper one measure of sodium carbonate, one
third measure of calcium oxide and a very small crystal of ferrous
ammonium sulfate. Place the mixture in your heating spoon, add five
drops of sodium silicate and stir contents with stirring rod. Use
your alcohol lamp and blowpipe to play the flame directly on the
mixture. Note the changes as the heating continues.
SUMMARY:
The green color of the glass is produced by ferrous ammonium
sulfate.
EXPERIMENT No. 223 Making Amber Glass
(CL-44, CL-55, CL-66, CL-77)
Repeat Experiment No. 222 substituting one half measure of powdered
charcoal for the ferrous ammonium sulfate.
EXPERIMENT No. 224 Making Dark Blue Glass
(CL-44, CL-55, CL-66, CL-77)
Repeat Experiment No. 222 substituting one crystal of cobalt
chloride for the ferrous ammonium sulfate.
120 SILICON
AND THE SILICATES
HOW TO WORK GLASS TUBING
The glass tubing supplied
in Lionel Chem-Lab sets is "soft" glass, melting at
quite a low temperature. Because you will need to construct
certain simple laboratory apparatus to perform some of the
experiments, you will have to know how to cut glass tubing, bend
it and smooth its sharp edges.
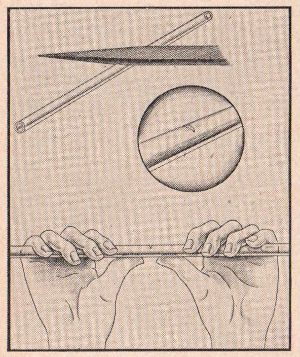
FIGURE 17
It is very easy to cut a piece of glass tubing
if you have a three-cornered file. Merely make a little scratch on
the glass with the file at the point where you wish to break the
tubing. Then grasp the tubing firmly with both hands, using your
thumbs as a fulcrum, and give it a sharp snap.
To smooth the sharp edges at the point of the
break, merely hold the end of the tube in the flame of your
alcohol lamp and rotate the tubing with your fingers. When the
glass gets hot enough it will begin to melt slightly which will
smooth the edges. This is sometimes called "fire-polishing".
To bend a piece of glass tubing, hold it in the
flame until the glass gets red hot at the point where you wish to
make the bend. Rotate the tube rapidly in your fingers so that
this section gets uniformly hot. You will feel and notice the
softening of the glass. When red hot, remove from the flame and
bend to the desired angle. Be careful, however, of heating until
it gets too soft otherwise the sides of the tubing will melt
together and close up the hole in the center.
It is also easy to make a tube with a nozzle,
such as an eyedropper. Cut off a section of glass tubing and hold
it in the flame until red hot, but instead if making the bend as
described in the preceding paragraph, stretch the tubing out until
it becomes very thin in the middle. Remove from the flame and make
a file cut at the place where the glass has the proper taper for
your particular purpose. Break off the extra piece of glass and
you will have the desired nozzle.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook