The
Science Notebook
Lionel Chem-Lab
- Chapter 14
The
Science Notebook
Lionel Chem-Lab
- Chapter 14
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 163 - 182
CHAPTER XIV
THE METALS
Of the ninety-two elements known to the
chemist, nearly four-fifths constitute a class of elements known
as the metals. The
majority of these are extremely rare and of little or no practical
importance. Only about fifteen are common, everyday substances
such as nickel, iron, copper, etc.
Although metals like gold and platinum are
found in the earth in the free state, most of the other members of
the metal family occur as minerals,
that is, in the earth combined with other elements. For example, iron oxide occurs as the
mineral, hematite, and
is the chief source of iron ore. Some of the minerals
supplied in Lionel Chem-Lab are: galena (lead sulfide), iron
pyrites (iron disulfide), stibnite (antimony trisulfide), feldspar
(potassium aluminum silicate), mica and talc. Metallurgy is the name given
to the science of extracting a metal from its ore.
TUNGSTEN AND MOLYBDENUM
Tungsten and molybdenum are two examples of the
rarer metals. Both have increased greatly in commercial importance
in the past few years. Tungsten wire is now used extensively in
our electric lights as a filament. Molybdenum compounds are used
to make dyestuffs and to color pottery. When alloyed w1th steel,
each contributes certain properties of hardness that makes it
important to the tool steel industries.
EXPERIMENT No. 352 The Reduction Of Tungstic Acid
(CL-77)
APPARATUS:
Sodium tungstate, sodium bisulfate, zinc metal, alcohol lamp and
test tube.
PROCEDURE:
Dissolve two measures of sodium tungstate in a test tube half full
of water. Add four measures of sodium bisulfate and a few pieces of
zinc metal. Shake test tube thoroughly. Heat the test tube and note
the deep blue color which is due to the formation of tungsten
pentachloride.
EXPERIMENT No. 353 Zinc Tungstate
(CL-77)
APPARATUS:
Zinc metal, three test tubes, sodium bisulfate, sodium tungstate,
alcohol lamp.
163
164 THE
METALS
PROCEDURE:
Place a small piece of zinc metal and three measures of sodium
bisulfate in a test tube one third full of water. Heat until a
portion of the zinc metal has dissolved and then pour the clear
solution into another test tube. Dissolve a measure of sodium
tungstate in a third test tube one half filled with water. Add a few
drops of the sodium tungstate solution to the zinc solution and note
the milky precipitate.
SUMMARY:
Zinc reacts with sodium bisulfate to form zinc sulfate and hydrogen.
The hydrogen escapes from the test tube in the form of a gas. The
zinc sulfate reacts with sodium tungstate to form the milky
precipitate of zinc tungstate.
California
Division of Mines
A tungsten
mill in which pure tungsten is prepared by converting it into
sodium tungstate and treating it with hydrochloric acid to
precipitate tungstic acid. The acid is reduced to pure tungsten
by mixing it with carbon and heating to a high temperature.
EXPERIMENT No. 354 Aluminum Tungstate
(CL-77)
APPARATUS:
Aluminum sulfate, sodium tungstate, two test tubes.
PROCEDURE:
Dissolve one measure of aluminum sulfate in a test tube one quarter
full of water. Dissolve one measure of sodium tungstate in another
test tube half full of water. Mix the two solutions and note the
thick white precipitate of aluminum tungstate.
LIONEL
CHEM-LAB 165
EXPERIMENT No. 355 Manganese Tungstate
(CL-77)
Repeat Experiment No. 354 substituting manganese sulfate for
aluminum sulfate. The precipitate will be manganese tungstate.
EXPERIMENT No. 356 Ferrous Tungstate
(CL-77)
Repeat Experiment No. 354 substituting ferrous ammonium sulfate for
aluminum sulfate. The precipitate will be ferrous tungstate.
EXPERIMENT No. 357 Chromium Tungstate
(CL-77)
Repeat Experiment No. 354 substituting chrome alum for aluminum
sulfate. The green precipitate will be chromium tungstate.
EXPERIMENT No. 358 Ferric Tungstate
(CL-77)
Repeat Experiment No. 354 substituting ferric chloride for aluminum
sulfate. The precipitate will be ferric tungstate.
EXPERIMENT No. 359 Copper Tungstate
(CL-77)
Repeat Experiment No. 354 substituting copper sulfate for aluminum
sulfate. The precipitate will be copper tungstate.
EXPERIMENT No. 360 Calcium Tungstate
(CL-77)
Repeat Experiment No. 354 substituting calcium chloride for aluminum
sulfate. The precipitate will be calcium tungstate.
EXPERIMENT No. 361 Cobalt Tungstate
(CL-77)
Repeat Experiment No. 354 substituting cobalt chloride for aluminum
sulfate. The pink precipitate will be cobalt tungstate.
EXPERIMENT No. 362 Strontium Tungstate
(CL-77)
Repeat Experiment No. 354 substituting strontium nitrate for
aluminum sulfate. The white precipitate will be strontium tungstate.
EXPERIMENT No. 363 How To Fireproof Materials
(CL-77)
APPARATUS:
Sodium tungstate, test tube, splint of wood, paper and cloth.
PROCEDURE:
Dissolve five measures of sodium tungstate in a test tube half full
of water. Drop in some small pieces of cloth, paper and a wooden
splint; remove and dry. Try to ignite these substances. Sodium
tungstate, a salt of tungsten, is used to fireproof materials.
166 THE
METALS
EXPERIMENT No. 364 Molybdic Acid Reduced
CL-77)
APPARATUS:
Ammonium molybdate, sodium bisulfate, zinc, alcohol lamp, test tube.
PROCEDURE:
Dissolve a measure of ammonium molybdate in a test tube half full of
water. Add four measures of sodium bisulfate and a small piece of
zinc. Shake well and heat gently. Note the blue color. Set the tube
aside for a while and note how the color gradually changes from blue
to green and finally to brown.
SUMMARY:
Zinc in the presence of hydrochloric acid colors a molybdate soluble
blue (due to the formation of molybdenum chloride), then green and
finally brown.
EXPERIMENT No. 365 Molybdenum Ferrocyanide
(CL-77)
APPARATUS:
Ammonium molybdate, sodium ferrocyanide, two test tubes.
PROCEDURE:
Dissolve half a measure of sodium ferrocyanide in a test tube one
quarter full of water. Dissolve one measure of ammonium molybdate in
another test tube half full of water. Mix the two solutions.
SUMMARY:
Ammonium molybdate reacts with sodium ferrocyanide to form a brown
precipitate of molybdenum ferrocyanide.
EXPERIMENT No. 366 A Solvent For Molybdenum
Ferrocyanide
(CL-77)
APPARATUS:
Ammonium hydroxide, ammonium molybdate, sodium ferrocyanide, test
tubes.
PROCEDURE:
Prepare a precipitate of molybdenum ferrocyanide as described in the
previous experiment. Add four drops of ammonium hydroxide to the
precipitate. Note that the precipitate dissolves forming a light
blue color.
EXPERIMENT No. 367 Molybdenum Trisulfide
(CL-77)
APPARATUS:
Ammonium molybdate, sodium bisulfate, sulfur, paraffin wax, delivery
tube, stopper and test tubes.
PROCEDURE:
Dissolve a measure of ammonium molybdate and an equal amount of
sodium bisulfate in a test tube half full of water. Put four
measures of sulfur and a small piece of paraffin into another test
tube; insert the delivery tube and stopper and heat gently to
generate hydrogen sulfide gas. Pass the gas into the ammonium
molybdate solution. Note the formation of a very dark precipitate of
molybdenum trisulfide.
LIONEL
CHEM-LAB 167
EXPERIMENT No. 368 Ferric Molybdate
(CL-77)
APPARATUS:
Ferric ammonium sulfate, ammonium molybdate and test tubes.
PROCEDURE:
Dissolve two measures of ferric ammonium sulfate in a test tube half
full of water. Dissolve one measure of ammonium molybdate in another
test tube half full of water. Pour a few drops of the ammonium
molybdate solution into the ferric ammonium sulfate solution and
note the formation of a thick yellow-brown precipitate of ferric
molybdate.
EXPERIMENT No. 369 Calcium Molybdate
(CL-77)
Repeat Experiment No. 368 substituting calcium chloride for ferric
ammonium sulfate. The white precipitate will be calcium molybdate.
EXPERIMENT No. 370 Ferrous Molybdate
(CL-77)
Repeat Experiment No. 368 substituting ferrous ammonium sulfate for
ferric ammonium sulfate. The dark brown precipitate will be ferrous
molybdate.
EXPERIMENT No. 371 Manganese Molybdate
(CL-77)
Repeat Experiment No. 368 substituting manganese sulfate for ferric
ammonium sulfate. The yellow precipitate will be manganese
molybdate.
EXPERIMENT No. 372 Molybdenum Tannate
(CL-77)
Repeat Experiment No. 368 substituting tannic acid for ferric
ammonium sulfate. The deep yellow color will indicate the presence
of molybdenum tannate in the solution.
EXPERIMENT No. 373 How To Make Molybdenum Blue
(CL.77)
APPARATUS:
Sodium bisulfate, ammonium molybdate, cane sugar, test tube, alcohol
lamp or candle.
PROCEDURE:
Mix four measures of cane sugar, two measures of ammonium molybdate
and two measures of sodium bisulfate in a test tube. Heat the
mixture gently. Remove test tube from flame when fumes form. Allow
to cool. Fill the test tube half full of water and note the color of
molybdenum blue.
168 THE
METALS
ALUMINUM
Aluminum, the most abundant of all the
metallic elements found in the earth’s crust, was at one time more
precious and rare than silver and gold. Only about 75 years ago,
the cost of aluminum per pound was $545. Today, under normal
conditions, the price is less than twenty cents a pound. Just how
it became inexpensive and universally used is a story of one of
the greatest industrial developments of modern times.
Aluminum is never found in the native state,
that is, as actual metal; instead it is always in chemical
combination with other elements. Its compounds are principally the
silicates and oxides, and while every clay
bank and practically all of our common rocks contain aluminum, bauxite is the only mineral
sufficiently rich in this metal to make its extraction economical.
Aluminum is silvery-white in color, very light
in weight, and an excellent conductor of heat and electricity.
Practically all branches of industry now use
aluminum in one form or another. Household utensils and
tooth-paste tubes are common uses in the home. Practically all
forms of transportation, streamlined trains, transport planes,
buses and trolley cars utilize aluminum when strong, lightweight
construction is desired.
EXPERIMENT No. 374 How To Make Aluminum Oxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Aluminum foil (store), potassium nitrate, test tube, candle or
alcohol lamp.
PROCEDURE:
Put three measures of potassium nitrate into a dry test tube and
apply heat until the crystals begin to melt. Drop the aluminum foil
into the tube. Note the burning.
SUMMARY:
Aluminum oxide is formed.
EXPERIMENT No. 375 How To Make Aluminum Hydroxide
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium hydroxide sol., aluminum sulfate, test tubes, alcohol lamp or
candle.
PROCEDURE:
Prepare some sodium hydroxide solution as explained in Experiment
No. 344. Dissolve one measure of aluminum. sulfate in a test tube
half full of water. Pour into this a few drops of the sodium
hydroxide solution and note the thick, white precipitate of aluminum
hydroxide.
EXPERIMENT No. 376 Another Way to Make Aluminum
Hydroxide
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Aluminum sulfate, sodium carbonate and test tubes.
PROCEDURE:
Dissolve one measure of aluminum sulfate in a test
LIONEL
CHEM-LAB 169
tube half full of water. Dissolve one measure of sodium carbonate in
another test tube half full of water. Mix the two solutions until a
white precipitate is formed.
EXPERIMENT No. 377 Aluminum Chromate
(CL-77)
APPARATUS:
Sodium chromate, aluminum sulfate and test tubes.
PROCEDURE:
Dissolve one half measure of sodium chromate in a test tube
containing one inch of water. In another test tube containing the
same amount of water, dissolve one measure of aluminum sulfate.
(Heat solution to dissolve crystals if necessary). Mix the two
solutions. A gelatinous orange precipitate of sodium [NOTE: This should be aluminum]
chromate is formed.
EXPERIMENT No. 378 Aluminum Acetate
(CL-44, CL-55, CL-66, GL-77)
APPARATUS:
Aluminum sulfate, acetic acid, test tubes, alcohol lamp or candle.
PROCEDURE:
Dissolve one measure of aluminum sulfate in a test tube half full of
water. Add five drops of acetic acid. Heat solution to boiling. Note
the cloudy precipitate of aluminum acetate formed.
EXPERIMENT No. 379 Testing For Aluminum
(CL-55, CL-66. CL-77)
APPARATUS:
Trisodium phosphate, aluminum sulfate and test tubes.
PROCEDURE:
Dissolve one measure of aluminum sulfate in a test tube half full of
water. Dissolve one measure of trisodium phosphate in another test
tube containing the same amount of water. Mix the two solutions. The
white precipitate is aluminum phosphate.
EXPERIMENT No. 380 Dissolving Aluminum Phosphate
(CL-55, CL-66, CL-77)
APPARATUS:
Trisodium phosphate, aluminum sulfate, hydrochloric acid and test
tubes.
PROCEDURE:
Dissolve one measure of aluminum sulfate in a test tube half full of
water. Dissolve one measure of trisodium phosphate in another test
tube half full of water. Mix the two solutions and a white
precipitate of aluminum phosphate will be formed. Now add a few
drops of hydrochloric acid. The precipitate dissolves due to the
formation of a soluble salt.
EXPERIMENT No. 381 Displacing Hydrogen From
Aluminum And Acid
(CL-11, CL-22, GL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, aluminum metal (store), test tube and wooden
splint.
170 THE
METALS
PROCEDURE:
Dissolve five measures of sodium bisulfate in a test tube one
quarter filled with water. Heat slowly and drop in a small piece of
aluminum metal. Place a lighted wooden splint near the mouth of the
tube.
SUMMARY:
A slight explosion is heard due to the combination of hydrogen and
air in the test tube.
EXPERIMENT No. 382 Testing For The Presence Of
Aluminum
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Aluminum sulfate, cobalt chloride, charcoal block, candle, test
tubes and blowpipe.
PROCEDURE:
Place a measure of cobalt chloride in a test tube one quarter filled
with water and shake to dissolve. Add a few drops of this to two
measures of aluminum sulfate powder and mix until a thin paste is
formed. Embed some of the paste in the charcoal block and heat by
directing the flame at it with the blowpipe. After a few moments the
paste will turn light blue indicating the presence of aluminum.
EXPERIMENT No. 383 Aluminum Hydroxide From
Aluminum Foil
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Hydrochloric acid, ammonium hydroxide (household ammonia), aluminum
foil and alcohol lamp.
PROCEDURE:
Pour about eight drops of hydrochloric acid in a test tube
containing some aluminum foil. Heat to boiling for one minute, then
add a few drops of ammonium hydroxide. Note the formation of a white
precipitate.
SUMMARY:
The hydrochloric acid reacts with aluminum to form aluminum chloride
which in turn is acted upon by the ammonium hydroxide to form the
white gelatinous precipitate, aluminum hydroxide.
MAGNESIUM
Magnesium, a light, silvery-white metal, burns
with a brilliant, white light and is used chiefly to make
fireworks and flashlight powders. In time of war, it is used to
make aerial flares. The chief sources of magnesium are the
minerals, dolomite and carnallite. Three of the
common compounds of magnesium are magnesium sulfate (Epsom salts), magnesium citrate and magnesium chloride.
EXPERIMENT No. 384 Magnesium Oxide
(CL-66, CL-77)
APPARATUS:
Magnesium sulfate, test tube, beaker or small glass, two carbon
rods. three dry cells in a series.
LIONEL
CHEM-LAB 171
PROCEDURE:
Dissolve five measures of magnesium sulfate in a test tube nearly
full of water. Heat almost to boiling, then transfer the solution to
a beaker or small glass. Insert two carbon rods as electrodes,
connecting one to the positive (plus) pole of the three cells in
series and the other to the negative (minus) pole. Keep the solution
warm during electrolysis. Note the gradual formation of a white
precipitate.
EXPERIMENT No. 385 Magnesium Hydroxide
(CL-66, CL-77)
APPARATUS:
Magnesium sulfate, sodium carbonate, calcium oxide, alcohol lamp or
candle, test tubes.
PROCEDURE:
Prepare some sodium hydroxide solution as explained in Experiment
No. 344. Dissolve two measures of magnesium sulfate in a test tube
half full of water. Add a few drops of the sodium hydroxide solution
and note the heavy white precipitate.
SUMMARY:
The heavy white precipitate is magnesium hydroxide, commonly known
as milk of magnesia.
EXPERIMENT No. 386 Magnesium Carbonate
(CL-66, CL-77)
APPARATUS:
Sodium carbonate, magnesium sulfate, test tubes.
PROCEDURE:
Dissolve one measure of sodium carbonate in a test tube one quarter
full of water. Dissolve one measure of magnesium sulfate in another
test tube containing the same amount of water. Add the sodium
carbonate solution to the magnesium sulfate. The white precipitate
is magnesium carbonate.
EXPERIMENT No. 387 Action Or Magnesium On Copper
(CL-66, CL-77)
APPARATUS:
Copper sulfate, magnesium sulfate and test tubes.
PROCEDURE:
Dissolve one measure of copper sulfate in a test tube half full of
water. Add one measure of magnesium sulfate.
SUMMARY:
Note the formation of a copper coating on the magnesium and the
disappearance of the characteristic green color of copper sulfate.
EXPERIMENT No. 388 Testing For Magnesium
(CL-66, CL-77)
APPARATUS:
Charcoal block, pen knife, cobalt chloride, magnesium sulfate,
blowpipe, alcohol lamp, eye dropper.
PROCEDURE:
Make a dent in the charcoal block to hold a small crystal of
magnesium sulfate. Moisten the sulfate with one drop of cobalt
chloride solution. Direct a flame on the charcoal block by means of
your blowpipe. Note the pale pink mass formed. This is a test for
magnesium.
172 THE
METALS
EXPERIMENT No. 389 Preparation Of Magnesium
Mixture
(CL-77)
APPARATUS:
Magnesium sulfate, ammonium chloride, ammonium hydroxide, test
tubes.
PROCEDURE:
Add one quarter measure of magnesium sulfate to a test tube one
quarter full of water. Add a few drops of ammonium hydroxide.
Dissolve one measure of ammonium chloride in another
test tube half full of water. Mix the two solutions. Magnesium
mixture is used when testing for the presence of a phosphate.
ZINC
Zinc, a bluish-white metal, occurs in nature
only in combined form. zinc
sulfate, or zinc
blende, is the most common ore of zinc.
One of the chief characteristics of zinc is its
tendency to react with the air to form a protective coating. It is
thus used for roofing, lining tanks and as a coating over sheet
iron known as galvanizing.
Industry today uses thousands of die cast parts made from zinc
alloy, an example of which is the door handle on your automobile -
cast of zinc alloy and then chromium plated. Other uses for zinc
are in the printing industry and the manufacture of paints and
electric batteries.
EXPERIMENT No. 390 Zinc Hydroxide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Zinc metal, sodium bisulfate, sodium carbonate, calcium oxide,
alcohol lamp or candle, test tube.
PROCEDURE:
Prepare some sodium hydroxide solution as described in Experiment
No. 344. Dissolve three measures of sodium bisulfate and a small
piece of zinc in a test tube half full of Water. Use heat if
necessary. Pour into this solution a few drops of sodium hydroxide
and note the white precipitate of zinc hydroxide.
EXPERIMENT No. 391 Preparing Cobalt Zincate
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Zinc, sodium carbonate, charcoal block, alcohol lamp, and cobalt
chloride.
PROCEDURE:
Make a dent in your charcoal block large enough to hold one measure
of sodium carbonate and one quarter measure of zinc. Direct a
blowpipe flame at the mixture. Add one or two small crystals of
cobalt chloride to the hot material. Reheat the mixture using the
blowpipe. A green mass of cobalt zincate is formed.
EXPERIMENT No. 392 Preparation Of Zinc
Sulfide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Zinc metal, hydrochloric acid, sulfur, paraffin, medicine dropper,
delivery tube, alcohol lamp or candle, test tube.
LIONEL
CHEM-LAB 173
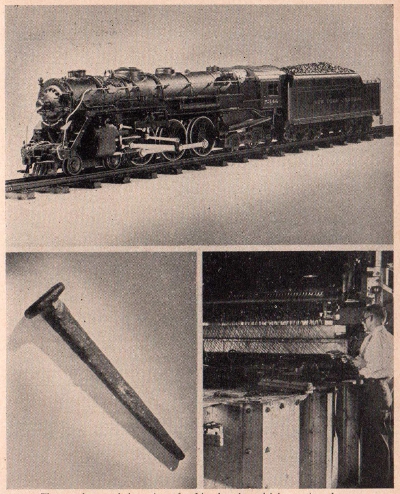
The top
photograph is a view of a Lionel scale-model locomotive, the
body of which is die-cast of zinc alloy. The iron nail shown at
lower left was coated with zinc and subjected to the severest of
weather conditions for over 55 years. Due to its heavy coating
of zinc, the iron did not rust. Lower right shows a two pan
galvanizing unit of the American Steel and Wire Company. This
complex machine is used for the purpose of covering wire with a
galvanized coating of zinc.
174
THE METALS
PROCEDURE:
Place five drops of hydrochloric acid in a test tube containing a
small piece of zinc metal. Heat the substance to boiling and set
aside until the bubbling reaction is complete. Fill the test tube
half full of water. Prepare some hydrogen sulfide gas as described
in Experiment No. 233. Allow the gas to bubble into the zinc
solution. Note the formation of a white precipitate.
SUMMARY:
Zinc reacts with hydrochloric acid to form hydrogen gas and zinc
chloride. The solution is set aside until the bubbling reaction is
complete because the hydrogen sulfide formed in the reaction between
sulfur and paraffin will not form a precipitate of zinc sulfide if
too much hydrogen is present.
EXPERIMENT No. 393 Zinc Sodium Ferrocyanide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Zinc powder, sodium ferrocyanide, test tubes, hydrochloric acid,
alcohol lamp or candle.
PROCEDURE:
Pour five drops of hydrochloric acid into a test tube containing one
measure of zinc powder. Heat the solution to boiling. Dissolve one
measure of sodium ferrocyanide in another test tube. Add about one
quarter test tube of zinc chloride (formed in the reaction between
hydrochloric acid and zinc) to the sodium ferrocyanide solution. The
white precipitate is zinc sodium ferrocyanide.
EXPERIMENT No. 394 Dissolving Zinc By A Weak Acid
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Vinegar, zinc, sodium ferrocyanide, test tube and alcohol lamp.
PROCEDURE:
Place a small piece of zinc in a test tube one quarter full of
vinegar. Heat gently and then set aside for an hour to dissolve the
zinc. Note the bubbling. Dissolve two measures of sodium
ferrocyanide in another test tube one quarter filled with water. Add
a few drops of the zinc solution to the sodium ferrocyanide solution
and note the white precipitate.
SUMMARY:
Zinc dissolves in the presence of acids liberating hydrogen gas,
which accounts for the bubbling reaction. The zinc in solution
reacts with sodium ferrocyanide to form the white precipitate of
zinc ferrocyanide.
EXPERIMENT No. 395 Displacing Hydrogen By Zinc
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Hydrochloric acid, zinc, test tube and alcohol lamp.
PROCEDURE:
Place one measure of zinc and four drops of hydrochloric acid in a
test tube. Heat test tube gently. Test the gas coming off by placing
the mouth of the test tube near the flame and note
LIONEL
CHEM-LAB 175
reaction. The slight explosion will be proof that hydrogen is being
displaced from the acid by the zinc.
EXPERIMENT No. 396 Fusing Zinc With Sodium
Carbonate
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Zinc, sodium carbonate, charcoal block, alcohol lamp and blowpipe.
PROCEDURE:
Make a dent in your charcoal block to hold one measure of sodium
carbonate and one quarter measure of zinc. Direct a blowpipe flame
at the mixture. When the substance is hot the color is yellow but
when it cools, the color changes to white.
CHROMIUM AND MANGANESE
Manganese and chromium are hard metals,
resembling steel in appearance, used principally in making steel
alloys. Large quantities of chromium are used in electro-plating
the bright finish on automobile trimmings and interior hardware.
Chrome alum, or chromium potassium sulfate as it is technically
known, is a lavender colored powder used principally in the
dyeing, leather and ink industries. We shall use this chemical in
performing the following experiments.
EXPERIMENT No. 397 Oxidation Of Chrome Alum
(CL-55, CL-66, CL-77)
APPARATUS:
Chrome alum, calcium hypochlorite, test tube.
PROCEDURE:
Dissolve one measure of chrome alum in a test tube half full of
water. Add two measures of calcium hypochlorite. Shake vigorously
and note the yellow color. Cautiously smell the odor coming from the
mouth of the tube. A yellow liquid will be formed and chlorine gas
given off.
EXPERIMENT No. 398 Chrome Alum And Sodium
Hydroxide Reaction
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Chrome alum, sodium carbonate, calcium oxide, test tubes.
PROCEDURE:
Prepare a solution of sodium hydroxide as described in Experiment
No. 344. Dissolve half a measure of chrome alum in half a test tube
of water. Add a little sodium hydroxide solution.
SUMMARY:
Note the green precipitate. (This precipitate is to be used for the
following experiments, therefore, do not destroy it.)
EXPERIMENT No. 399 Sodium Chromate
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Hydrogen peroxide (drug store), precipitate previously prepared by
you, test tubes.
176 THE
METALS
PROCEDURE:
Add a few drops of hydrogen peroxide to the precipitate prepared in
the preceding experiment. The yellow precipitate is sodium chromate
frequently used as a paint pigment.
EXPERIMENT No. 400 Chrome Alum And Ammon1um
Hydroxide
(CL-77)
APPARATUS:
Chrome alum, ammonium hydroxide, candle or alcohol lamp, test tubes.
PROCEDURE:
Dissolve one half measure of chrome alum in a test tube containing a
tablespoonful of water. Add a few drops of ammonium hydroxide. Note
the green gelatinous precipitate formed. Now add ammonium hydroxide
until the precipitate dissolves. A slightly pink colored solution
will be formed. Heat to the boiling point and see that a new
precipitate appears.
SUMMARY:
The first green precipitate was chromium hydroxide. Upon the
addition of an excess of ammonium hydroxide solution, the
precipitate dissolves. A precipitate again appears upon boiling.
EXPERIMENT No. 401 Sodium Carbonate And Chrome
Alum
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, chrome alum and test tubes.
PROCEDURE:
Dissolve one half measure of chrome alum in a test tube half full of
water. Dissolve one half measure of sodium carbonate in another test
tube one quarter filled with water. Add the sodium carbonate to the
chrome alum solution and note the pale green precipitate.
EXPERIMENT No. 402 How To Make Chromium Phosphate
(CL-55, CL-66, CL-77)
APPARATUS:
Chrome alum, trisodium phosphate and test tubes.
PROCEDURE:
Dissolve one half measure of chrome alum in a test tube half filled
with water. Dissolve one half measure of trisodium phosphate in
another test tube containing three quarters of an inch of water. Mix
the two solutions together in a third test tube.
SUMMARY:
A green precipitate of chromium phosphate is formed. If a dilute
acid is added, the precipitate will dissolve.
EXPERIMENT No. 403 Testing For Chromium
(CL-66, CL-77)
APPARATUS:
Sodium carbonate, chrome alum, nichrome wire, alcohol lamp.
PROCEDURE:
Form a loop in the end of your nichrome wire. Moisten the loop in
water. Mix one quarter measure of chrome alum and one measure of
sodium carbonate on a sheet of paper. Dip the moistened wire loop
into this mixture. Place the wire in the oxidizing portion of
LIONEL
CHEM-LAB 177
the flame. The flame should be yellow indicating the presence of
chromium.
EXPERIMENT No. 404 A Blowpipe Test For
Chromium
(CL-44, GL-55, CL-66, CL-77)
APPARATUS:
Charcoal, blowpipe, alcohol lamp, pen knife, sodium carbonate,
chrome alum.
PROCEDURE:
Place one measure of sodium carbonate and a few crystals of chrome
alum in a small dent on your charcoal block. Direct the blowpipe
flame at this substance. The green mass is a test for chromium used
in blowpipe analysis.
EXPERIMENT No. 405 A Test For Chromium Compounds
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Nichrome wire, boric acid, chrome alum, alcohol lamp.
PROCEDURE:
Prepare a borax bead as explained in Experiment No. 491. Add a small
piece of chrome alum to the bead. Place bead in the center cone of
the flame.
SUMMARY:
The borax bead is white but when chrome alum is added and the whole
heated over an oxidizing flame, a green colored bead is formed. This
is an excellent test for chrome.
MANGANESE
EXPERIMENT No. 406 Preparation Of Manganese
Hydroxide
(CL-77)
APPARATUS:
Manganese sulfate ammonium hydroxide, test tubes.
PROCEDURE:
Dissolve one measure of manganese sulfate in a test tube half full
of water. Add a few drops of ammonium hydroxide. Note the
flesh-colored precipitate. Set aside for one hour. Note how the
color changes to brown.
SUMMARY:
Upon exposure to air, the precipitate changes to a brown color,
because a mixture of manganic hydroxide and manganous acid is
formed.
EXPERIMENT No. 407 Manganese Hydroxide
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Manganese sulfate, sodium carbonate, calcium oxide, test tube,
alcohol lamp or candle.
PROCEDURE:
Prepare some sodium hydroxide solution as explained in Experiment
No. 344. Dissolve two measures of manganese sulfate in a test tube
half full of water. Add a few drops of sodium hydroxide solution and
note the thick white precipitate.
178 THE
METALS
SUMMARY:
This precipitate (which gets darker on standing) is manganese
hydroxide.
EXPERIMENT No. 408 Preparation Of Manganese
Tungstate
(CL-77)
APPARATUS:
Sodium tungstate, manganese sulfate, test tubes.
PROCEDURE:
Dissolve one measure of manganese sulfate in a test tube half full
of water. Dissolve one half measure of sodium tungstate in another
test tube containing the same amount of water. Add some sodium
tungstate solution to the manganese sulfate. The white precipitate
is manganese tungstate.
EXPERIMENT No. 409 Preparation Of Manganese
Phosphate
(CL-77)
APPARATUS:
Manganese sulfate, trisodium phosphate, ammonium hydroxide, test
tubes.
PROCEDURE:
Dissolve one measure of trisodium phosphate in a test tube half full
of water. Add three drops of ammonium hydroxide. Dissolve half a
measure of manganese sulfate in another test tube one quarter full
of water. Mix both solutions and note the flesh-colored precipitate
due to the presence of ammonium hydroxide in the solution.
EXPERIMENT No. 410 D1ssolving Manganese Phosphate
(CL-77)
APPARATUS:
Manganese sulfate, ammonium hydroxide, ammonium chloride, test
tubes.
PROCEDURE:
Dissolve one measure of ammonium chloride in a test tube half full
of water. Dissolve one measure of manganese sulfate in another test
tube containing the same amount of water. Add two drops of ammonium
hydroxide to the latter solution. Note the precipitate formed. Now
add the ammonium chloride solution and note that the precipitate
dissolves.
EXPERIMENT No. 411 Preparation Of Manganese
Carbonate
(CL-55, CL-66, CL-77)
APPARATUS:
Manganese sulfate, sodium carbonate and two test tubes.
PROCEDURE:
Dissolve two measures of manganese sulfate in a test tube half full
of water. Dissolve one measure of sodium carbonate in another test
tube half full of water. Mix the two solutions and note the
formation of a white precipitate.
SUMMARY:
The manganese sulfate reacts with the soluble sodium carbonate to
form the white precipitate of manganese carbonate. This is an
example of double decomposition.
LIONEL
CHEM-LAB 179
EXPERIMENT No. 412 How To Make Manganese
Molybdate
(CL-77)
APPARATUS:
Ammonium molybdate, manganese sulfate, test tubes.
PROCEDURE:
Dissolve half a measure of ammonium molybdate in a test tube one
quarter full of water. Dissolve one half measure of manganese
sulfate in another test tube containing the same amount of Water.
Pour the ammonium molybdate solution into the manganese sulfate
solution. The yellow solution is manganese molybdate.
EXPERIMENT No. 413 A Test For Manganese
(CL-66, CL-77)
APPARATUS:
Nichrome wire, sodium carbonate, manganese sulfate, alcohol lamp.
PROCEDURE:
Make a loop about one sixteenth of an inch in diameter at the end of
your nichrome wire. Moisten the loop in water, then dip it into some
sodium carbonate. Direct the flame from your blowpipe at the mass.
Continue to heat and re-dip until the loop is entirely filled with a
sodium carbonate bead. Insert the hot bead into a small portion of
manganese sulfate and again apply heat, making certain that the
manganese sulfate is dissolved by the sodium carbonate bead.
SUMMARY:
Compare the different colors of the bead when heated by the
oxidizing and a reducing flame. Clean wire by heating and carefully
flipping the bead out, then dipping it into water to remove the
particles.
EXPERIMENT No. 414 Testing For Manganese
Compounds
(CL-66, CL-77)
APPARATUS:
Boric acid, manganese sulfate, nichrome wire, sheet of clean paper,
alcohol lamp.
PROCEDURE:
Make a borax bead as described in Experiment No. 491. Add a small
amount of manganese sulfate to the bead. Place the bead in the
oxidizing portion of the flame.
SUMMARY:
Note that when the bead is hot the color of the solid is violet but
when cool, the color changes to amethyst red.
EXPERIMENT No. 415 Fusing Manganese Sulfate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Manganese sulfate, sodium carbonate, potassium nitrate, a piece of
porcelain, alcohol lamp, blowpipe.
PROCEDURE:
Place one measure of potassium nitrate and one measure of sodium
carbonate in a piece of porcelain. Add a few crystals of manganese
sulfate. Direct the blowpipe flame at this mixture. The green mass
which is formed is due to the presence of manganese.
180 THE
METALS
TIN
Tin is chiefly used as a protective coating
for other metals. The familiar tin can, as most people know, is
not really made of tin at all but sheet iron, tin-plated. Tin
foil, used in packaging foods, is practically pure tin. The metal
is also used as an alloy in making solder and type
metal.
EXPERIMENT No. 416 Tin Chloride
(CL-55, CL-66, CL-77)
APPARATUS:
Tin metal, hydrochloric acid, test tube, sodium ferrocyanide,
alcohol lamp or candle, eye dropper.
PROCEDURE:
Add a few drops of hydrochloric acid to a test tube containing a
small piece of tin metal. Note reaction, if any. Heat carefully
(holding the tube away from the face). Allow it to boil for a few
minutes. Pour off the clear solution into another test tube.
Dissolve one measure of sodium ferrocyanide into a third test tube
half filled with water. Now add a few drops of the tin chloride
solution. Note the formation of a light blue-green precipitate.
SUMMARY:
Dilute hydrochloric acid has very little effect on cold tin.
However, when heated, some of the tin goes into solution to form tin
chloride. This combines with sodium ferrocyanide to form the
bluish-green precipitate of tin ferrocyanide.
EXPERIMENT No. 417 How To Make Stannic Hydroxide
(CL-55, CL-66, CL-77)
APPARATUS:
Tin chloride (prepared in the previous experiment), calcium oxide,
sodium carbonate, test tubes.
PROCEDURE:
Prepare a solution of sodium hydroxide as described in Experiment
No. 344. Add seven drops of sodium hydroxide solution to the tin
chloride solution. Note the precipitate.
SUMMARY:
The white precipitate is tin hydroxide. (If you have added more than
the amount of sodium hydroxide specified the precipitate may not
form because tin hydroxide is soluble in excess sodium hydroxide
solution.)
EXPERIMENT No. 418 Preparation Of Tin Sulfide
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tin metal, hydrochloric acid, test tube, sulfur, paraffin, delivery
tube, stopper, candle or alcohol lamp.
PROCEDURE:
Place five drops of hydrochloric acid in a test tube containing a
small piece of tin metal. Heat test tube for a few minutes. Remove
test tube from flame and when cool, add an inch of water. Prepare
some hydrogen sulfide gas as described in Experiment No. 233. Bubble
gas through the delivery tube into the tin solution. The precipitate
is stannic sulfide.
LIONEL
CHEM-LAB 181
EXPERIMENT No. 419 The Properties Of Tin
(CL-11. CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tin metal, hammer and alcohol lamp.
PROCEDURE:
Test the properties of tin by pounding a small piece or a hard
surface with a hammer. Hold a piece of tin over a flame and note how
readily it melts.
EXPERIMENT No. 420 Preparation Of Tin Crystals
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tin plate, hydrochloric acid.
PROCEDURE:
Place five drops of dilute hydrochloric acid on a piece of tin
plate. Allow to dry, then rinse with water. Note the crystals of tin
chloride.
LEAD
Galena,
or lead sulfide, is the
principal mineral from which lead is extracted.
Lead, the heaviest of the common metals and
very soft and malleable, is used in making white lead for paint,
lead plates for storage batteries, and for underground pipes and
protective tubes.
EXPERIMENT No. 421 Testing Galena For Lead
(CL-77)
APPARATUS:
Galena, hydrochloric acid, test tube holder and test tube.
PROCEDURE:
Put one small piece of galena into a test tube. Add ten drops of
hydrochloric acid. Boil contents for a few minutes. Set aside and
note that a white precipitate forms upon cooling.
SUMMARY:
When galena is heated with hydrochloric acid, and then the solution
is cooled, a precipitate of lead chloride forms. (The precipitate
will not form in the hot solution).
EXPERIMENT No. 422 Testing Galena For Sulfide
(CL-77)
APPARATUS:
Galena, hydrochloric acid, test tube holder and test tube.
PROCEDURE:
Place a small piece of galena in a test tube. Add ten drops of
hydrochloric acid. Boil contents for a few minutes. (Note the
bubbling reaction which takes place). Moisten a piece of lead
acetate paper and place it at the mouth of the test tube. Note the
color of the paper.
SUMMARY:
Galena (lead sulfide) when heated with hydrochloric acid forms
hydrogen sulfide which colors lead acetate black.
182 THE
METALS
EXPERIMENT No. 423 How To Make Lead Chromate
(CL-77)
APPARATUS:
Galena, hydrochloric acid, sodium chromate, test tube and test tube
holder.
PROCEDURE:
Prepare a solution of lead chloride as described in Experiment No.
421. Dissolve one half measure of sodium chromate in a test tube one
quarter full of water. Add the chromate to the lead chloride and
note that an orange precipitate of lead chromate is formed.
EXPERIMENT No. 424 Preparing Lead Sulfate From
Galena
(CL-77)
APPARATUS:
Galena, hydrochloric acid, sodium sulfate, test tube and holder.
PROCEDURE:
Prepare a solution of lead chloride as explained in Experiment No.
421. Prepare a solution of sodium sulfate by dissolving one measure
of sodium sulfate in a test tube one quarter full of water. Add
eight drops of sodium sulfate to the lead chloride solution. Note
the color of the precipitate which is lead sulfate.
EXPERIMENT No. 425 Preparing Lead Iod1de
(CL-77)
APPARATUS:
Galena, hydrochloric acid, sodium iodide, test tubes and holder.
PROCEDURE:
Prepare a solution of lead chloride as described in Experiment No.
4-21. Dissolve one measure of sodium iodide in a test tube one
quarter full of water. Add the sodium iodide to the lead chloride
and note the orange-yellow, crystalline precipitate of lead iodide.
EXPERIMENT No. 426 How To Make Lead Ferrocyanide
(CL-77)
APPARATUS:
Galena, hydrochloric acid, sodium ferrocyanide, test tubes and
holder.
PROCEDURE:
Prepare a solution of lead chloride as explained in Experiment No.
421. Dissolve one measure of sodium ferrocyanide in a test tube one
quarter full of water. Add the sodium ferrocyanide to the lead
chloride and note that a white precipitate of lead ferrocyanide is
formed.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook