The
Science Notebook
Lionel Chem-Lab
- Chapter 15
The
Science Notebook
Lionel Chem-Lab
- Chapter 15
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 183 - 190
CHAPTER XV
THE STORY OF IRON AND STEEL
Iron has been known and widely used as a metal
for over four thousand years. Its greatest use today is in the
manufacture of steel, an alloy of iron and carbon capable of being
hardened, toughened and otherwise altered by suitable heat
treatment. With the exception of the meteorites which fall to the
earth, iron does not occur free in nature. However, the ores of
iron are very abundant and the metal is easily produced from them.
The foremost iron ores are magnetite,
hematite, limonite and a carbonate
known as siderite.
Pure iron has very few uses and is rarely
produced. When other elements such as carbon are added, even in
small percentages, the properties of iron are considerably changed
and it becomes much more useful.
Cast iron
is made by mixing iron ore with a suitable flux and reducing it by
heating with coke. This operation is carried out in a large tower
eighty feet high and twenty feet in diameter, known as a blast furnace.
STEEL
Steel is made from cast iron by burning out a
part of the carbon, silicon, phosphorous and sulfur. The chief
processes for doing this are the Bessemer Process and the Open-Hearth Process.
When the manufacture of steel is finished, the
molten metal is poured into molds for castings, or cast into
ingots to be forged into bars, rails, and other suitable forms for
commercial and industrial purposes.
The alloy steels have other elements than
carbon added in proper percentages to give them exceptional
qualities of hardness and toughness. The chief elements used in
these alloying processes are chromium, cobalt, tungsten,
molybdenum, manganese and nickel.
Nickel steels are the most important alloy
steels. Adding chromium to the extent of 12% or 13% makes steel
completely rust-proof. It is then commercially known as stainless steel.
EXPERIMENT No. 427 Ferrous Hydroxide
(CL-55, GL-66, GL-77)
APPARATUS:
Ferrous ammonium sulfate, sodium carbonate, calcium oxide, test tube
and alcohol lamp or candle.
PROCEDURE:
Prepare some sodium hydroxide solution as explained in Experiment
No. 344. Dissolve one measure of ferrous ammonium
183
184 IRON
AND STEEL
sulfate in a test tube half full of water. Add a few drops of sodium
hydroxide and note the green precipitate of ferrous hydroxide which
darkens upon standing.
EXPERIMENT No. 428 Another Way To Make
Ferrous Hydroxide
(CL-77)
APPARATUS:
Ferrous ammonium sulfate, ammonium hydroxide, test tube.
PROCEDURE:
Dissolve one half measure of ferrous ammonium sulfate in a test tube
half full of water. Add a few drops of ammonium hydroxide. Note the
dark green precipitate of ferrous hydroxide.
EXPERIMENT No. 429 Ferrous Ammonium Molybdate
(CL-77)
APPARATUS:
Ammonium molybdate, ferrous ammonium sulfate and test tubes.
PROCEDURE:
Dissolve one half measure of ammonium molybdate in a test tube
containing one inch of water. Dissolve one measure of ferrous
ammonium sulfate in another test tube half full of water. Add
some ammonium molybdate to the ferrous ammonium sulfate solution.
The brown-black precipitate is ferrous ammonium molybdate.
EXPERIMENT No. 430 Ferrous Ammonium Molybdate
Dissolved
(CL-77)
APPARATUS:
Ammonium molybdate, ferrous ammonium sulfate, test tubes,
hydrochloric acid.
PROCEDURE:
Prepare ferrous ammonium molybdate as described in the preceding
experiment. Add five drops of hydrochloric acid to the precipitate.
Note how the precipitate dissolves and the resulting color.
EXPERIMENT No. 431 Preparing Ferrous Chromate
(CL-77)
APPARATUS:
Sodium chromate, ferrous ammonium sulfate and test tubes.
PROCEDURE:
Dissolve one half measure of sodium chromate in a test tube one
quarter full of water. Dissolve one measure of ferrous ammonium
sulfate in another test tube half full of water. Mix the two
solutions. The orange-brown precipitate is ferrous chromate.
EXPERIMENT No. 432 Dissolving Ferrous Chromate
(CL-77)
APPARATUS:
Sodium chromate, hydrochloric acid, ferrous ammonium sulfate and
test tubes.
PROCEDURE:
Dissolve a half measure of sodium chromate in a test
LIONEL
CHEM-LAB 185
U.
S. National Museum
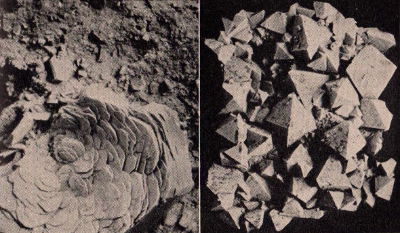
An
illustration of two of the variety of forms in which iron ore
occurs. The photograph on the left shows the hexagonal crystals
of hematite while the one on the right shows the rhombic
crystals of Swiss magnetite.
tube one quarter full of water. Dissolve one measure of ferrous
ammonium sulfate in another test tube half full of water. Mix the
two solutions. An orange-brown precipitate of ferrous chromate is
formed. Now add a few drops of hydrochloric acid and the precipitate
will dissolve because a soluble salt was formed.
EXPERIMENT No. 433 How To Make Ferrous Sulfide
(CL-77)
APPARATUS:
Ammonium hydroxide, ferrous ammonium sulfate, paraffin, sulfur,
candle or alcohol lamp.
PROCEDURE:
Prepare some hydrogen sulfide as described in Experiment No. 233.
Dissolve one measure of ferrous ammonium sulfate in a test tube half
full of water. Pass some hydrogen sulfide gas into the above
solution. Note that a black precipitate of ferrous sulfide is formed
which, upon exposure to air, oxidizes and converts the ferrous
sulfide into ferric sulfide, a brown precipitate.
EXPERIMENT No. 434 How To Make Ferrous Salicylate
(CL-55, CL-66, CL-77)
APPARATUS:
Ferrous ammonium sulfate, sodium salicylate, test tubes.
PROCEDURE:
Dissolve one measure of ferrous ammonium sulfate in a test tube
containing one inch of water. Dissolve one measure of sodium
salicylate in another test tube containing the same amount of
186 IRON AND STEEL
water. Mix the two solutions to obtain ferrous salicylate, a
rust-colored solution.
EXPERIMENT No. 435 How To Make Ferrous Tungstate
(CL-77)
APPARATUS:
Ferrous ammonium sulfate, sodium tungstate, test tubes.
PROCEDURE:
Dissolve one measure of ferrous ammonium sulfate in a test tube half
full of water. Dissolve one half measure of sodium tungstate in
another test tube one quarter full of water. Mix the two solutions.
The light-brown precipitate is ferrous tungstate.
EXPERIMENT No. 436 Oxidizing Ferrous Hydroxide
(CL-77)
APPARATUS:
Ammonium hydroxide, hydrogen peroxide (drug store), ferrous ammonium
sulfate and test tubes.
PROCEDURE:
Prepare a precipitate of ferrous hydroxide by using the ammonium
hydroxide reagent. (Experiment No. 428). Add exactly three drops of
hydrogen peroxide. Note the color change in the precipitate.
SUMMARY:
Hydrogen peroxide, a fairly strong oxidizing agent when added to a
ferrous salt, oxidizes it to the ferric state, and the color changes
from green to reddish-brown.
EXPERIMENT No. 437 Reducing Ferric To Ferrous
Chloride
(CL-66, CL-77)
APPARATUS:
Ferric chloride, ferrous ammonium sulfate, hydrochloric acid, zinc,
gas generator bottle, alcohol lamp or candle, test tubes.
PROCEDURE:
Dissolve one measure of ferric chloride in a test tube containing
one inch of water. Prepare some hydrogen gas as described in
Experiment No. 72. Bubble some gas into the above solution and note
that hydrogen is capable of reducing ferric to ferrous chloride.
EXPERIMENT No. 438 Ferric Chloride And Ammonium
Hydroxide
(01.-66, cL-77)
APPARATUS:
Ferric chloride, ammonium hydroxide and test tubes.
PROCEDURE:
Dissolve one measure of ferric chloride in a test tube one quarter
full of water. Add a few drops of ammonium hydroxide.
SUMMARY:
Ferric chloride reacts with ammonium hydroxide to form ammonium
chloride and ferric hydroxide (reddish-brown precipitate).
EXPERIMENT No. 439 Slow Oxidation Of Iron Pyrites
(CL-66, CL-77)
APPARATUS:
Iron pyrites, blue litmus paper, saucer.
LIONEL
CHEM-LAB 187
PROCEDURE:
Pour just enough water into a saucer to cover three measures of iron
pyrites. Set aside for a few days. Dip piece of blue litmus paper
into the water. Note the change in color.
SUMMARY:
Blue litmus paper changes in color due to the formation of hydrogen
sulfide - the iron being slowly oxidized to iron oxide by oxygen in
the water.
EXPERIMENT No. 440 Ferric Hydroxide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, sodium carbonate, calcium oxide, alcohol
lamp or candle and test tubes.
PROCEDURE:
Prepare some sodium hydroxide solution as explained in Experiment
No. 344. Dissolve two measures of ferric ammonium sulfate in a test
tube half full of water. Add a few drops of sodium hydroxide
solution. The reddish-brown precipitate, which is similar to iron
rust, is ferric hydroxide.
EXPERIMENT No. 441 Dissolving Ferric Hydroxide
(CL-77)
APPARATUS:
Hydrochloric acid, ferric chloride, ammonium hydroxide and test
tubes.
PROCEDURE:
Dissolve one measure of ferric chloride in a test tube one quarter
full of water. Add a few drops of ammonium hydroxide to obtain a
reddish-brown precipitate. Add a few drops of hydrochloric acid.
Note how the precipitate dissolves in an acid solution proving that
ferric hydroxide is soluble in acids.
EXPERIMENT N0. 442 Transforming Ferric Hydroxide
(CL-77)
APPARATUS:
Ferric chloride, ammonium hydroxide, test tubes, alcohol lamp.
PROCEDURE:
Prepare a precipitate of ferric hydroxide as described in Experiment
No. 440. Heat precipitate thoroughly for about five minutes. Note
the color formation of the solid. This is ferric oxide known as
rouge in the cosmetics industry.
EXPERIMENT No. 443 How To Make Ferric Tungstate
(CL-77)
APPARATUS:
Sodium tungstate, ferric chloride and test tubes.
PROCEDURE:
Dissolve one measure of ferric chloride in a test tube half full of
water. Add one measure of sodium tungstate to another test tube
containing the same amount of water. Add one half the amount of
sodium tungstate solution to the ferric chloride solution. The pale
yellow precipitate is ferric tungstate.
188 IRON
AND STEEL
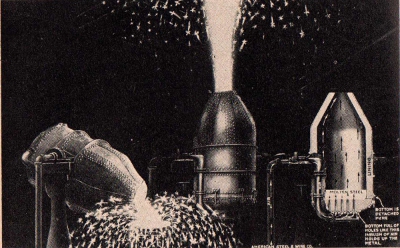
In the
Bessemer process of steel-making, a. blast of compressed air is
forced through molten pig iron to convert it into steel.
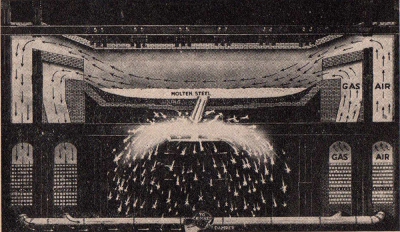
Ore of
less purity than that used in the Bessemer process is
successfully made into fine steel by the Open Hearth process.
LIONEL
CHEM-LAB 189
EXPERIMENT No. 444 Preparation Ferric Chromate
(CL-77)
APPARATUS:
Ferric chloride, sodium chromate and test tubes.
PROCEDURE:
Dissolve one half measure of sodium chromate in a test tube one
quarter full of water. Dissolve one measure of ferric chloride in
another test tube half full of water. Mix the two solutions. The
pale yellow precipitate is ferric chromate, soluble in hydrochloric
acid.
EXPERIMENT N0. 445 How To Make Ferric Salicylate
(CL-66, CL-77)
Repeat Experiment No. 444 substituting sodium salicylate for sodium
chromate. The purple precipitate will be ferric salicylate.
EXPERIMENT No. 446 Preparation Of Ferric
Phosphate
(CL-66, GL-77)
Repeat Experiment No. 444 substituting trisodium phosphate for
sodium chromate. The yellowish-white precipitate will be ferric
phosphate.
EXPERIMENT No. 447 Preparation Of Ferric Acetate
(CL-66, CL-77)
APPARATUS:
Calcium oxide, sodium carbonate, acetic acid, ferric chloride and
test tubes.
PROCEDURE:
Prepare a solution of sodium hydroxide as described in Experiment
No. 344. Filter solution in a clean test tube and add one quarter
test tube of acetic acid solution. Shake the test tube thoroughly.
Dissolve one measure of ferric chloride in another test tube. Add
some acetate solution to the ferric chloride solution.
SUMMARY:
A reddish-brown color is formed. This color is attributed to the
formation of the complex salt of ferric acetate.
EXPERIMENT No. 448 How Ferric Carbonate Is Made
(CL-66, CL-77)
Repeat Experiment No. 44-44 substituting sodium carbonate for sodium
chromate. The yellow-orange precipitate will be ferric carbonate.
EXPERIMENT No. 449 Ferric Molybdate
{CL-77)
Repeat Experiment No. 444 substituting ammonium molybdate for sodium
chromate. The pale yellow precipitate will be ferric molybdate.
EXPERIMENT N0. 450 Converting A Yellow
Precipitate
(CL-77)
APPARATUS:
Ferric chloride, ammonium molybdate, hydrochloric acid and test
tubes.
190 IRON
AND STEEL
PROCEDURE:
Prepare a precipitate of ferric molybdate explained in the preceding
experiment. Add four drops of hydrochloric acid to the precipitate.
Note how the precipitate dissolves forming a light yellow-green
solution.
EXPERIMENT No. 451 Free Carbon Present In Cast
Iron
(CL-22, CL~33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered iron, sodium bisulfate, test tube, candle or alcohol lamp.
PROCEDURE:
Put one measure of powdered iron and five measures of sodium
bisulfate in a test tube. Pour water up to the one third mark and
heat gently to dissolve the sodium bisulfate. Note the bubbling
reaction and the odor.
SUMMARY:
Carbon in the iron is oxidized to carbon dioxide gas. The odor is
hydrogen sulfide.
EXPERIMENT No. 452 Preparing A Colored Solution
(CL-66, CL-77)
APPARATUS:
Ferric carbonate, sodium carbonate, hydrochloric acid and test
tubes.
PROCEDURE:
Prepare a precipitate of ferric carbonate as described in Experiment
No. 448. Add a few drops of hydrochloric acid to the precipitate.
Note how the precipitate dissolves forming a canary-yellow solution.
EXPERIMENT No. 453 Decomposition Of Ferric
Thiosulfate
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium thiosulfate, ferric ammonium sulfate, two test tubes.
PROCEDURE:
Dissolve three measures of sodium thiosulfate in a test tube half
full of water. Dissolve one measure of ferric ammonium sulfate in
another test tube half full of water. Pour a few drops into the
sodium thiosulfate solution and note the dark red color. Allow to
stand and observe how the color gradually fades.
SUMMARY:
On standing the ferric is reduced to ferrous tetrathionate, a
colorless solution.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook