The
Science Notebook
Lionel Chem-Lab
- Chapter 20
The
Science Notebook
Lionel Chem-Lab
- Chapter 20
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 211 - 220
CHAPTER XX
INKS
Inks for writing purposes have been known for
thousands of years. In ancient days they were black and, like
modern India ink, derived their color from carbon. When tannic
acid, a product of nut galls, combines with iron sulfate, a
substance known as ferrous
tannate is formed which is the essential constituent of
ordinary black ink. Other substances such as hydrochloric and
carbolic acids and a suitable dye are added to make up the ink for
writing purposes. Ferrous tannate is nearly colorless, but when
the ink is exposed to the air, the ferrous salt is oxidized to a
ferric salt which then develops the black iron compound. A blue
dye is usually added to give immediate color to the writing. This
is why ordinary fountain pen ink first appears blue but changes to
black.
EXPERIMENT No. 517 How Commercial Writing Ink Is
Prepared
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tannic acid, sodium ferrocyanide, ferric ammonium sulfate, test tube
and pen.
PROCEDURE:
Dissolve one measure of tannic acid and one measure of sodium
ferrocyanide in a test tube half full of water. Dissolve two
measures of ferric ammonium sulfate in another test tube one quarter
full of water. Mix the two solutions thoroughly. Try writing with
this ink, adding a little water if the ink is not free flowing.
Preserve the ink, if you wish, by adding a few drops of carbolic
acid.
EXPERIMENT No. 518 A Special Black Ink Powder
(CL-66, CL-77)
APPARATUS:
Tannic acid, ferric ammonium sulfate, mortar and pestle.
PROCEDURE:
Place one measure of tannic acid and one measure of ferric ammonium
sulfate into the mortar. Mix thoroughly by grinding. Put the powder
into a container and add water whenever
black ink is needed.
EXPERIMENT No. 519 A Formula For A Blue Writing
Ink
(CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric chloride, two test tubes.
PROCEDURE:
Dissolve two measures of sodium ferrocyanide in a test tube one
quarter full of water. Dissolve one measure of ferric
211
212 INKS
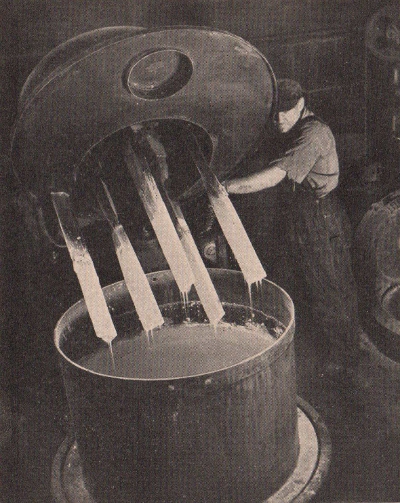
International
Printing Ink Co.
Varnishes, dryers
and pigments for printers ink are mixed in this large "dough
mixer" which has a capacity of about 300 pounds.
LIONEL
CHEM-LAB 213
chloride in another test tube one quarter full of water. Mix the two
solutions and note the blue color. This ink is Prussian blue, often
used in the dye industry.
EXPERIMENT No. 520 Blue Ink Powder
(CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric ammonium sulfate, mortar and pestle.
PROCEDURE:
Place two measures of sodium ferrocyanide and one measure of ferric
ammonium sulfate in the mortar. Grind the contents into a fine
powder. Put this powder into a container and add water whenever blue
ink is needed.
EXPERIMENT No. 521 Canary Yellow Ink
(CL-77)
APPARATUS:
Tannic acid, sodium chromate, test tube.
PROCEDURE:
Place one half measure of sodium chromate in a test tube. Dissolve
one measure of tannic acid in another test tube one quarter full of
water. Pour the tannic acid solution into the sodium chromate. Shake
test tube thoroughly for one minute and a canary yellow ink will
result.
EXPERIMENT No. 522 Making Yellow Ink Brown
(CL-77)
APPARATUS:
Tannic acid, sodium chromate, manganese sulfate and test tubes.
PROCEDURE:
Follow the same procedure as in the preceding experiment and add one
measure of manganese sulfate. Note the color change.
SUMMARY:
Manganese sulfate when treated with sodium chromate and tannic acid
produces a cocoa-brown color.
EXPERIMENT No. 523 A Secret Formula
(CL-77)
APPARATUS:
Copper sulfate, sodium chromate, tannic acid and test tubes.
PROCEDURE:
Place one measure of copper sulfate and one half measure of sodium
chromate in a test tube. Dissolve two measures of tannic acid in
another test tube one quarter full of water. Add the tannic acid
solution to the mixture. Note the resulting color.
SUMMARY:
Copper sulfate produces a yellowish-green ink when treated with a
solution of tannic acid and sodium chromate.
214 INKS
EXPERIMENT No. 524 A Green Ink
(CL-77)
APPARATUS:
Copper sulfate, sodium chromate, ammonium hydroxide and test tubes.
PROCEDURE:
Prepare yellowish green ink as described in the preceding
experiment. Add two drops of ammonium hydroxide. Note the resulting
color. The yellowish-green ink is turned to a solid green when
ammonium hydroxide is added.
EXPERIMENT No. 525 Preparing Moss-green Ink
(CL-77)
APPARATUS:
Tannic acid, sodium chromate, magnesium sulfate and test tubes.
PROCEDURE:
Place one measure of magnesium sulfate and. one half measure of
sodium chromate in a test tube. Dissolve two measures of tannic acid
in another test tube one quarter full of water. Add the tannic acid
solution to the above mixture and note the color of the ink.
SUMMARY:
Magnesium sulfate when treated with tannic acid and sodium chromate
produces moss-green ink.
EXPERIMENT No. 526 Carmine Writing Ink
(CL-77)
APPARATUS:
Cochineal, funnel, filter paper and writing pen.
PROCEDURE:
Place three measures of cochineal in a test tube half full of water
and boil until the solution becomes deep red. Try writing with this
solution, then filter and collect the red ink in another tube. To
preserve, add a few drops of carbolic acid.
EXPERIMENT No. 527 Mixing Two Different Colors
(CL-77)
APPARATUS:
Cochineal, galena, hydrochloric acid, sodium chromate, alcohol lamp,
test tubes.
PROCEDURE:
Prepare a solution of lead chloride as described in Experiment No.
421. Dissolve one half measure of sodium chromate in a test tube one
quarter full of water. Now add the lead chloride solution. Dissolve
one measure of cochineal in another test tube one-quarter full of
water. Add the cochineal to the chromate solution and note the
brilliant yellow color.
EXPERIMENT No. 528 Formula For Violet Ink
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Logwood, aluminum sulfate, test tube, alcohol lamp or candle.
PROCEDURE:
Place two measures of logwood in a test tube one
LIONEL
CHEM-LAB 215
quarter full of water and heat for several minutes. Dissolve half a
measure of aluminum sulfate in the solution and a violet ink will
result.
SUMMARY:
Violet ink is obtained.
EXPERIMENT No. 529 How To Make Loowood Ink
(CL-44, CL-55. CL-66, CL-77)
APPARATUS:
Logwood, sodium carbonate, test tubes, alcohol lamp or or candle.
PROCEDURE:
Dissolve two measures of sodium carbonate in a test tube one quarter
full of water. Put one measure of logwood in another test tube one
quarter full of water and boil for a few minutes. Add the extract to
the sodium carbonate solution. A dark brown ink will result.
EXPERIMENT No. 530 Brown Writing Ink
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, copper sulfate and test tube.
PROCEDURE:
Dissolve one measure of copper sulfate in a test tube half full of
water. Add one measure of sodium ferrocyanide and shake well. Brown
colored ink will result.
EXPERIMENT No. 531 How To Make Gold Inks
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Bronze powder, honey, calcium oxide and sodium carbonate.
PROCEDURE:
Prepare a solution of sodium hydroxide as described in Experiment
No. 344. Place two measures of bronze powder in the mortar. Add two
drops of sodium hydroxide and five drops of honey. Grind until it
becomes a fine gold paste which may be diluted to proper consistency
by adding sodium hydroxide solution.
EXPERIMENT No. 532 A Secret Formula For Green Ink
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric ammonium sulfate, test tube and some
barberry twigs.
PROCEDURE:
Place two or three measures of barberry shavings into a test tube
containing half an inch of water. Heat until a yellow color sets in.
Boil for a minute then add one measure of sodium ferrocyanide and a
small crystal of ferric ammonium sulfate and a green ink will result
.
216
INKS
EXPERIMENT No. 533 Black Ink
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tannic acid, ferric ammonium sulfate and test tube.
PROCEDURE:
Dissolve one half measure of tannic acid in a test tube one quarter
full of water. Dissolve one half measure of ferric ammonium sulfate
in a test tube one quarter full of water. Mix the two solutions to
obtain a good black writing ink.
EXPERIMENT No. 534 A Formula For Red Ink
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, logwood, aluminum sulfate, test tube, alcohol lamp
or candle.
PROCEDURE:
Place one measure of logwood in a test tube one quarter full of
water. Heat until the color is extracted. Add one half measure of
aluminum sulfate and one measure of sodium bisulfate, then shake to
dissolve the materials. This makes a good red ink.
EXPERIMENT No. 535 A Brilliant Red Ink
(CL-77)
APPARATUS:
Cochineal, ammonium hydroxide, alcohol lamp and test tubes.
PROCEDURE:
Put one measure of cochineal in a test tube one quarter full of
water. Heat test tube to boiling, then remove from flame and add
five drops of ammonium hydroxide.
SUMMARY:
When cochineal is treated with ammonium hydroxide, a brilliant red
color is produced.
EXPERIMENT No. 536 Red Pink Powder
(CL-77)
APPARATUS:
Cochineal, mortar and pestle.
PROCEDURE:
Place three measures of cochineal in your mortar. Grind thoroughly
until it becomes a fine powder. Put this into a container and add
water whenever red ink is needed.
EXPERIMENT No. 537 Printing Ink
(CL-66, CL-77)
APPARATUS:
Charcoal powder, sodium ferrocyanide, ferric chloride, test tubes.
PROCEDURE:
Dissolve one measure of sodium ferrocyanide in a test tube one
quarter full of water. Dissolve one measure of ferric chloride in
another test tube containing the same amount of water. Mix the two
solutions. Add two measures of charcoal powder. Stir
LIONEL
CHEM-LAB 217
the materials with your glass stirring rod until a smooth, even
emulsion is formed. This ink will be similar to that used by
printing industry.
EXPERIMENT No. 538 How To Prepare Safety Ink
(CL-66, CL-77)
APPARATUS:
Sodium silicate, powdered charcoal, mortar and pestle.
PROCEDURE:
Place one measure of powdered charcoal in the mortar. Add one
spoonful of sodium silicate solution. Stir and mix thoroughly. A
black, indelible ink will result. The name "safety ink" is applied
to any ink which makes erasing and alteration difficult.
EXPERIMENT No. 539 Making Another Type Or Safety
Ink
(CL-66, CL-77)
APPARATUS:
Tannic acid, ferric ammonium sulfate, sodium silicate solution,
powdered charcoal, mortar and pestle.
PROCEDURE:
Dissolve one measure of tannic acid and an equal amount of ferric
ammonium sulfate in a test tube one quarter full of water. Add a
spoonful of sodium silicate solution. Place the mixture in the
mortar together with two measures of powdered charcoal. Grind
thoroughly with the pestle. Note the indelible black ink.
EXPERIMENT No. 540 Decomposition Of Ink
(CL-77)
APPARATUS:
Ammonium hydroxide, logwood, copper sulfate, alcohol lamp and test
tubes, aluminum sulfate.
PROCEDURE:
Place one measure of logwood in a test tube one quarter full of
water. Heat the solution to extract color from the wood. Dissolve
one measure of aluminum sulfate in another test tube containing one
inch of water. Add four drops of ammonium hydroxide to the aluminum
sulfate. Heat test tube to boiling for a minute or two. Set aside
until cool, then add the logwood extract and note the color
formation. Dissolve one measure of copper sulfate in another test
tube one quarter full of water. Add the copper sulfate to the above
compound and note how the color of the solution is transformed.
SUMMARY:
Ammonium alum plus logwood produces a violet color ink. This ink
decomposes slowly upon the addition of copper sulfate to form a
blue-black color.
EXPERIMENT No. 541 An Ink Remover
(CL-55, CL-66, CL-77)
APPARATUS:
Tartaric acid, calcium hypochlorite, test tube and small, soft
brush.
218 INKS
PROCEDURE:
Dissolve one half measure of tartaric acid and one half measure of
calcium hypochlorite in a test tube containing a half inch of water.
Brush some of this solution over some writing done with ordinary
ink. Note how the writing disappears.
A number of "secret" inks can be prepared which
when heated or exposed to dry air become visible. `
EXPERIMENT No. 542 A Secret Formula For
Sympathetic Ink
(CL-66, CL-77)
APPARATUS:
Rice, iodine (drug store), mortar and pestle.
PROCEDURE:
Place three measures of rice in your mortar and grind it to a fine
powder. Place one small crystal of iodine in a test tube one quarter
full of water. Shake test tube to dissolve the crystal and then add
the rice powder. Using this ink, write on a piece of paper and note
how the writing disappears. (It will reappear when warmed). This
sympathetic ink was used during the Indian Mutiny to conceal
messages.
EXPERIMENT No. 543 Making Invisible Ink
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, test tube, white paper and pen.
PROCEDURE:
Dissolve two measures of cobalt chloride in a test tube half full of
water. Write on a sheet of white paper with this ink and note that
the writing is invisible. Heat the paper carefully and note how the
writing appears in blue. The writing gradually disappears as the
paper is cooled.
EXPERIMENT No. 544 An Unusual Ink
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Starch mixture, iodine crystals (drug store), writing pen, test
tubes, and small camelís hair brush.
PROCEDURE:
Dissolve one iodine crystal in a test tube one quarter full of
water. Dip your pen into some starch mixture and write on a piece of
paper. (Prepare starch mixture according to Experiment No. 688). Let
the writing dry, then brush iodine solution over it lightly.
Starch produces an invisible writing which will appear if
brushed with iodine solution.
EXPERIMENT No. 545 Another Secret Ink
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Lemon, writing pen and paper.
PROCEDURE:
Squeeze some lemon juice into a test tube. Dip the
LIONEL
CHEM-LAB 219
pen into the juice and write your name on a piece of paper. Note how
the name eventually disappears. Place the paper in a warm place and
note that the name reappears.
SUMMARY:
Lemon juice was used during the World War to send secret messages.
EXPERIMENT No. 546 Invisible Ink From Milk
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Milk, pen, paper, alcohol lamp.
PROCEDURE:
Write a secret message using ordinary milk as ink. Note that the
writing gradually disappears on standing. Put the paper in a warm
place and the writing will reappear.
EXPERIMENT No. 547 Restoring A Sympathetic Ink
(CL-66, CL-77)
APPARATUS:
Nickel chloride, lemon juice, pen, paper, test tube and small
camelís hair brush.
PROCEDURE:
Dip pen into some lemon juice and write your name on a clean sheet
of paper. Dissolve two measures of nickel chloride in a test tube
half full of water. Dip the brush into the solution of nickel
chloride and wash lightly over the lemon juice writing. Note that
the letters are permanently restored.
EXPERIMENT No. 548 How To Make A Durable
Sympathetic Ink
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, pen and white paper.
PROCEDURE:
Dissolve five measures of sodium bisulfate in a test tube one
quarter full of water. Write with this ink on a sheet of white paper
and note that the writing is not visible. Place the paper near a
flame and note that the writing reappears.
EXPERIMENT No. 549 How To Take Fingerprints
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric ammonium sulfate, test tube, sheet of
white paper and wad of cotton.
PROCEDURE:
Dissolve two measures of sodium ferrocyanide in a test tube one
quarter full of water. Dissolve one measure of ferric ammonium
sulfate in a test tube one quarter full of water. Mix the two
solutions, and note the blue coloring. Moisten the ball of the left
index finger with this liquid, wiping off any excess with cotton.
Press your finger on a sheet of paper rolling the finger from left
to right to get a clean impression. By following the same procedure,
you can make prints of all your fingers.
The science of fingerprint identification is
one of the important phases of modern criminology.
220
INKS
EXPERIMENT No. 550 A Blue Fingerprint Ink
(CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric chloride and test tube.
PROCEDURE:
Dissolve two measures of sodium ferrocyanide in a test tube one
quarter full of water. Dissolve one measure of ferric chloride in
another test tube one quarter full of water. Note the blue ink
suitable for taking fingerprints.
EXPERIMENT No. 551 Discovering Fingerprints On
Dark Objects
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, dark glass bottle, talcum powder.
PROCEDURE:
Mix three measures of sulfur with six measures of white talcum
powder. Handle a dark bottle until it is covered with your
fingerprints. Dust some of the mixture evenly over the fingerprints
and shake off the excess quantity. Warm the bottle gently to "set"
the prints. This is how the police detect fingerprints on dark
surfaces.
EXPERIMENT No. 552 Discovering Fingerprints On
Light Objects
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Powdered charcoal, sulfur, a piece of white paper.
PROCEDURE:
Mix four measures of powdered charcoal with an equal amount of
sulfur. Breathe on your right index finger several times and then
press it on the piece of paper. Note that the print is not visible.
Dust some of the mixture over the paper and shake off the excess.
Note that the fingerprint is now visible.
SUMMARY:
Heat the paper cautiously over a flame to preserve the print.
EXPERIMENT N0. 553 How To Make Typewriter Ribbon
(cr.-sc, CL-77)
APPARATUS:
Glycerine, ferric chloride, sodium ferrocyanide, test tubes, mortar
and pestle.
PROCEDURE:
Put five drops of glycerine and twenty measures of soap into your
mortar. Dissolve one measure of ferric chloride and one measure of
sodium ferrocyanide in a test tube one quarter full of water. Pour
this solution into the mortar and stir until a smooth paste forms.
Spread the paste smoothly on a narrow strip of white cloth and allow
to dry for one half hour.
SUMMARY:
This ink is easily absorbed by cloth and may be used in typewriter
ribbons.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook