The
Science Notebook
Lionel Chem-Lab
- Chapter 22
The
Science Notebook
Lionel Chem-Lab
- Chapter 22
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 229 - 234
CHAPTER XXII
TEXTILES AND PAPER
Carbohydrate
is the name applied to a group of carbon compounds which includes
sugars, starches and cellulose. These compounds
all contain carbon, hydrogen and oxygen, the last two being
present in the ratio of two to one, just as in water. Cellulose
having the formula C6H10O5 is an
example of the relationship.
All plants, wood and vegetable fibers contain
cellulose either free or combined. Cotton and linen are nearly
pure cellulose. Paper, another common cellulose product, is
manufactured from wood pulp or rags. Cellulose can be separated
from wood and vegetable fibers by a strong base or acid.
There are various grades and qualities of paper
ranging from cheap, porous substances, such as newsprint, to fine
stationery and bonds made from rags. Newsprint is made primarily
from spruce wood pulp. All papers, whether made from wood pulp or
rags, are essentially cellulose derivatives.
EXPERIMENT No. 583 How Rag Paper Is Made
(CL-66, CL-77)
APPARATUS:
Sodium carbonate, calcium oxide, test tube, mortar and pestle, bits
of rag, alcohol lamp or candle.
PROCEDURE:
Put a few small pieces of rag in a test tube. Add four measures of
sodium carbonate and three measures of calcium oxide. Add water
until the test tube is three quarters full, then carefully boil for
a few minutes, taking care not to spill the liquid. Transfer
contents to the mortar and grind well until a pulp is formed. Rinse
the pulp by pouring off the old water and adding new water. Remove
as much water as possible and set the pulp aside to dry. This
process is similar to that used in making rag-content writing paper.
EXPERIMENT No. 584 How Wood Pulp Paper Is Made
(CL-66, CL-77)
APPARATUS:
Sodium bisulfate, match, mortar and pestle, test tube, alcohol lamp
or candle and a dish.
PROCEDURE:
Carefully whittle live or six match sticks (without heads) and four
measures of sodium bisulfate into your mortar. Grind the shavings
with the pestle until they are reduced to shreds. Place the contents
in a test tube one third full of water and boil for
229
230
TEXTILES AND PAPER
du Pont
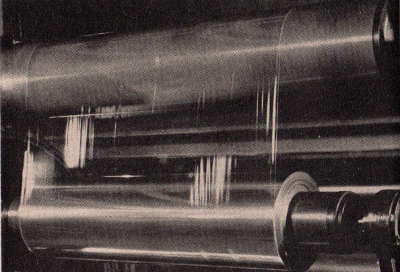
The upper
photograph shows a shredding machine in which sheets of wood
pulp are shredded after having been treated with a solution of
caustic soda. This is the first step toward the production of
finished "Cellophane" which is shown in the lower picture being
wound on cores at tremendous speed.
LIONEL
CHEM-LAB 231
five minutes, taking care not to let the liquid spill over. Transfer
the contents of the tube to the mortar and grind well until a pulp
is formed. Rinse the pulp by pouring off the old water and adding
new water. Remove as much water as possible and place the pulp on a
dish to dry. Examine it carefully. This material will closely
resemble the pulp used in making newsprint.
EXPERIMENT No. 585 Testing Paper For Starch
(CL-44, CL-55, CL-66, GL-77)
APPARATUS:
Ferric ammonium sulfate, sodium bisulfate, sodium iodide solution,
paper and test tube.
PROCEDURE:
Dissolve one measure of ferric ammonium sulfate and one measure of
sodium bisulfate in a test tube half full of water. Add two drops of
sodium iodide solution and shake well. Note the orange-brown color.
Apply a little of this solution to a sheet of paper. Reverse the
paper and note if a blue stain appears. The starch test can be used
to prove that paper contains starch.
CELLOPHANE
Cellophane and rayon are similar in that both
are made from cellulose. In the case of rayon, a cellulose
solution is forced through microscopic holes into a chemical bath
which changes the tiny streams of “liquid cellulose" back into
filaments of solid cellulose. In the manufacture of cellophane,
the viscose solution is forced out into the chemical bath through
a long narrow slit and the result is a thin film of cellulose.
TEXTILE FIBERS
Cotton and linen yarns, composed principally
of cellulose, have a vegetable source, but wool and silk yarns are
made from animal fibers. Vegetable and animal fibers look very
much alike at first glance, but by feeling them, examining them
closely, applying certain chemical tests to them, or looking at
the fibers under a microscope, certain essential differences in
the structure and shape of the fiber soon appear. These are
natural textile fibers. In the last few years, chemistry has
developed a whole group of new synthetic materials, such as rayon
and nylon, which have completely revolutionized the textile and
garment industry and have supplanted the natural fibers in many
articles of clothing.
EXPERIMENT No. 586 Odor Test For Vegetable Fibers
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Vegetable fibers (cotton or linen), candle or alcohol lamp.
PROCEDURE:
Burn the vegetable fibers over the candle. Note the
232
TEXTILES AND PAPER
faint odor, similar to burning paper. Note how these fibers burn
rapidly leaving white ashes. White ashes and the odor of burning
paper are characteristics of burning vegetable fibers.
EXPERIMENT No. 587 How Cloth Is Tested For Animal
Fibers
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Silk and wool, candle or alcohol lamp.
PROCEDURE:
Burn first some silk then some wool fibers. Note the peculiar odor
similar to that of burning hair. Notice how long the fibers burn and
also the ball of carbon which remains. A burned hair odor is a test
for animal fibers.
EXPERIMENT No. 588 A Test For Rayon
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Piece of rayon cloth, candle or alcohol lamp.
PROCEDURE:
Unravel some fibers from a piece of rayon and burn them. Note the
presence of any odor.
SUMMARY:
Rayon made from an acetic acid compound will possibly give a
slightly irritating sensation to your nose when burned.
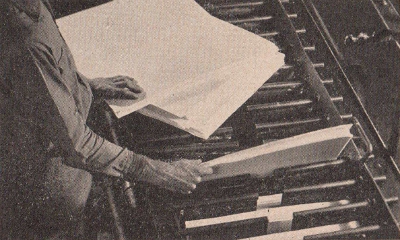
The first
step in the manufacture of rayon. The operator is shown here
loading a steeping press with sheets made of cotton linters and
wood pulp.
LIONEL
CHEM-LAB 233
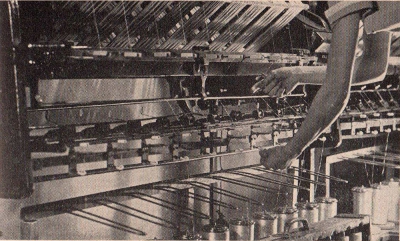
A du Pont rayon reeling
machine. The operator is drawing rayon threads through guides so
they may be wound into skeins on the spindles below.
EXPERIMENT No. 589 How Fabrics Are Tested For
Pure Linen Fibers
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, glycerine, test tube, linen, candle or alcohol
lamp and two blotters.
PROCEDURE:
Dissolve one half measure of sodium bisulfate in a test tube half
full of water. Drop a piece of linen into this solution, then boil
for a few minutes. Remove linen from test tube and wash thoroughly.
Set aside to dry. Expose the fibers by raveling the edges, then
dampen one corner with a drop of glycerine. Squeeze the cloth
between two blotters and place it against a dark background. Note
whether the saturated corner of the cloth is translucent. The purity
of linen is proved by its translucency.
EXPERIMENT No. 590 An Alkali Test For Animal
Fibers
(CL-11, GL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, sodium carbonate and a few strands of white wool
yarn.
PROCEDURE:
Make some sodium hydroxide by dissolving two measures of calcium
oxide and two measures of sodium carbonate in a test tube half full
of water. Heat to boiling for a few minutes, insert the wool and
continue to boil for a few minutes longer. If the material
234
TEXTILES AND PAPER
dissolves completely, it is pure wool. Check this test by performing
the same experiment with some cotton fibers.
EXPERIMENT No. 591 Testing New Cotton Goods For
Starch
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, ferric ammonium sulfate, sodium iodide solution,
two test tubes and cotton goods.
PROCEDURE:
Dissolve one measure of sodium bisulfate and one half measure of
ferric ammonium sulfate in a test tube half full of water. Add a
drop of sodium iodide solution. Put a small piece of cotton goods
into another test tube half full of water and boil for a few
minutes. Remove the cotton and allow the liquid to cool. Add two
drops of sodium iodide solution and note any change in color. If a
blue color forms, the material contains starch.
EXPERIMENT No. 592 Rejuvenating Old Silk
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Small piece of old silk, acetic acid, glass, blotters.
PROCEDURE:
Pour a small quantity of acetic acid into a glass. Place the silk in
the acid and allow it to steep for five or ten minutes. Remove the
silk and place it between the blotters, squeezing out most of the
acid. Allow the silk to dry and then note how its luster and
characteristic rustle have been restored. Acetic acid is popular
with chemists for dissolving organic substances and in dilute form
it is frequently used by tailors and cleaners for restoring old
silks.
NYLON
Nylon should be considered not as a single
compound, nor as assuming any particular form, such as yarn.
Rather, nylon is the name of a family of materials, like wood,
steel, stone or glass, any one of which may take various forms.
Just as we have different kinds of glass, designed particularly
for windows, spectacle lenses, or cooking-vessels, so are there
many different types of nylon, each with its individual
properties. Think of a mysterious chemical family that can produce
monofilaments for brush bristles, surgical sutures and
fishing-leaders; fibers for sheer hosiery, dress goods, bathing
suits, draperies and upholstery; insulation for electrical wires;
and sheets rivaling leather in strength and toughness!
Nylon’s most extensive use now is in fine
hosiery. Because of the natural abundance of its basic ingredients
- coal, air and water - it promises to make the hosiery industry
less and less dependent on foreign raw materials.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook