The
Science Notebook
Lionel Chem-Lab
- Chapter 26
The
Science Notebook
Lionel Chem-Lab
- Chapter 26
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 283 - 288
CHAPTER XXVI
CHEMISTRY OF SOILS AND FERTILIZERS
Soil is chiefly a product of the weathering of
rock and consequently varies greatly in composition and physical
properties. "Weathering of rock" refers to the natural action of
the weather: rain, wind and air - which have a very powerful
grinding effect on rock structure.
Limestone soil results from the disintegration
of limestone. Other soils of sandy and clay type are rich in
silicates. Acidity is also a characteristic of certain soils. This
is due to the decaying of plant and vegetable matter in the ground
and the subsequent forming of acid products. Inasmuch as crops do
not thrive on such soil, it becomes necessary for the chemist to
devise a satisfactory method for keeping the soil sweet. This the
farmers in many sections of the country do by using calcium
hydroxide or ground limestone to neutralize the acid condition.
FERTILIZERS
Plants derive their nourishment from both soil
and air, but the soil is the more important of the two. It is
vital, therefore, that land for agricultural purposes contain the
proper constituents to encourage the growth of plant life. When a
plant grows, it utilizes certain food elements in the soil and
thus in order to maintain the fertility of the soil in subsequent
years, it becomes necessary to return these elements to the
ground. This is the purpose of fertilizers.
In our chapter on nitrogen we emphasized the
importance of the nitrates to plant growth. Nitrogenous material
is one of the most important materials used to maintain the
fertility of the soil. Phosphates of calcium and potassium are
other frequently used ingredients.
Nitrogenous matter may be obtained from a
number of different sources, principally “natura1" fertilizers or
the waste products of animals, refuse from slaughterhouses, and
pure chemical compounds such as calcium cyanamide and sodium
nitrate.
Due to the scarcity of many of the nitrates,
the converting of the natural nitrogen in the air by the process
known as the fixation of
nitrogen promises in the future
to supply more and more of our requirements for nitrogenous
compounds.
Calcium
phosphate is an important ingredient used in the manufacture of
fertilizers. Small quantities can be obtained by grinding bones.
The prin-
283
284 SOILS
AND FERTILIZERS
cipal source, however, is the rich phosphate
quarries in Florida and Tennessee where phosphate rock is actually
mined from the ground.
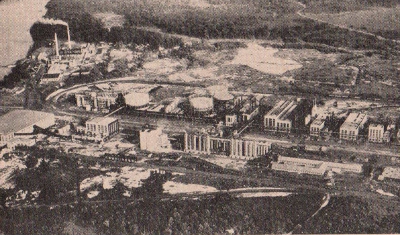
An air
view of a huge nitrate plant in Hopewell, Virginia. It is here
that great quantities of nitrate of soda are produced to be used
to restore the fertility of farm fields sterilized by repeated
crop cultivation.
EXPERIMENT No. 778 A Mineral Fertilizer
(CL-77)
APPARATUS:
Sodium borate, trisodium phosphate, ammonium chloride, aluminum
sulfate, ferric ammonium sulfate, manganese sulfate, ammonium
hydroxide, magnesium sulfate, calcium carbonate, potassium
carbonate, a container, and measuring spoon.
PROCEDURE:
Place in a large container one measure of sodium borate, six
measures of trisodium phosphate, two measures of ammonium chloride,
one measure of aluminum sulfate, one measure of ferric ammonium
sulfate, one half measure of manganese sulfate, six drops of
ammonium hydroxide, one measure of magnesium sulfate and one measure
of calcium carbonate. Pour into this one quart of warm water and two
measures of potassium carbonate. Sprinkle this over the house
plants. After a reasonable time, an improvement will be noted in the
general condition of these plants.
EXPERIMENT No. 779 Making A Garden Fertilizer
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
LIONEL
CHEM-LAB 285
APPARATUS:
Wood ash, poultry manure and tin can.
PROCEDURE:
Mix in a clean tin can two parts of wood ash with one part of
poultry manure. Insert a tablespoonful of this into the soil around
the roots of a leafy garden plant. Within a reasonable time the
general condition of the plant will improve.
EXPERIMENT No. 780 How Carbon Dioxide Affects
Plant Growth
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Two mason jars, soil, beans, vinegar, baking soda, a milk bottle,
drinking glass and teaspoon.
PROCEDURE:
In each mason jar, place two inches of damp soil. Then plant three
or four beans in each jar. Prepare some carbon dioxide in a quart
milk bottle by mixing two teaspoonfuls of baking soda with a half
glass of vinegar. Transfer the carbon dioxide to one of the jars by
pouring it. Be careful not to transfer any of the liquid. Label this
jar so as to distinguish it from the other jar which does not
contain carbon dioxide. Seal both jars and set aside, noting from
time to time
the jar in which the beans grow faster.
SUMMARY:
Since green plants need carbon dioxide to manufacture food, the
plant with the larger supply will grow the faster.
EXPERIMENT No. 781 Testing Soil For Alkalinity
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Test tube, soil to be tested, red litmus paper and saucer.
PROCEDURE:
Mix some soil in a saucer with enough water to form a thin paste.
Insert a strip of red litmus paper and set aside for a half hour.
Remove paper and rinse thoroughly. Note whether there is a change in
color. If the soil is alkaline the red litmus paper will become
blue.
EXPERIMENT No. 782 How To Test Soil For Acidity
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Soil, test tube, blue litmus paper and saucer.
PROCEDURE:
Mix a little soil in the saucer with enough water to form a thin
paste. Insert a strip of blue litmus paper and set aside for a half
hour. Remove the paper and rinse it thoroughly. Note whether there
is a change in color. If the soil is acid, the blue litmus paper
will become red.
INSECTICIDES
EXPERIMENT No. 783 A Solution Of Lime Sulfur
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, sulfur, glass, test tube and a candle or alcohol
lamp.
286 SOILS
AND FERTILIZERS
The
Barrett Company
Even the Staff of
Life may be improved upon by chemistry. The wheat which is used
to produce such high quality bread as shown above greatly
diminishes the fertility of American soil each year. Through
modern chemistry, this fertility is restored continually by the
application of nitrate of soda. The lower photograph shows the
difference between a crop treated with nitrate and one left
untreated.
LIONEL
CHEM-LAB 287
PROCEDURE:
Mix four measures of calcium oxide and two measures of sulfur in a
test tube. Fill the tube halfway with water and boil until the
solution becomes yellow. Dilute the solution with a half glass of
water and use it as a spray on fruit trees. This solution is an
effective insecticide.
EXPERIMENT No. 784 An Oil-Emulsion Insecticide
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, sulfur, test tube, thin motor oil, naphtha soap,
candle or alcohol lamp and a beaker.
PROCEDURE:
Mix two measures of calcium oxide and one measure of sulfur in a
test tube. Fill the tube halfway with water and boil until the
solution becomes yellow, Pour the solution into a beaker containing
four teaspoonfuls of motor oil. Add a small piece of naphtha soap
and boil until an emulsion forms. Dilute this with a half pint of
water. This emulsion may be used to destroy "sucking" insects which
are harmful to green plants.
EXPERIMENT No. 785 Sulfur As A Fungicide
(CL-66, CL-77)
APPARATUS:
Sulfur, mortar and pestle, paint brush.
PROCEDURE:
Grind a teaspoonful of sulfur in the mortar. Apply this powder to a
mildewed plant with a paint brush. After a reasonable time the
sulfur should destroy the mildew. However, in severe cases,
application may have to be made more than once.
EXPERIMENT No. 786 Testing Bordeaux Mixture For
Free Copper
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, calcium oxide, test tubes, candle or alcohol lamp,
beaker, vinegar, sodium ferrocyanide, funnel and filter paper.
PROCEDURE:
Place one measure of copper sulfate in a test tube one half full of
water and heat to dissolve. Put four measures of calcium oxide in
another test tube one half full of water and boil for a short time.
Cool both test tubes and pour the solutions into a beaker or glass
and stir. Filter a little of this solution into a clean test tube.
Add four drops of vinegar. Place one half measure of sodium
ferrocyanide in another test tube one quarter full of water and
shake well. Add a few drops of this to the filtered mixture and note
any color change.
SUMMARY:
If the solution becomes reddish-brown, copper is present. The copper
in Bordeaux mixture destroys fungi but does not injure plants.
EXPERIMENT No. 787 Making A Copper Fungicide
(CL-55, CL-66, CL-77)
288
SOILS AND FERTILIZERS
APPARATUS:
Copper sulfate, calcium oxide, two test tubes, alcohol lamp or
candle and a beaker.
PROCEDURE:
Place one measure of copper sulfate in a test tube one half full of
water and heat to dissolve. Place four measures of calcium oxide in
another test tube one half full of water and boil for a short time.
Cool both test tubes and pour the solutions into a beaker or glass
and stir. Set this solution aside and note the precipitate which
forms. The fungicide is used to kill fungi on grapevines.
EXPERIMENT No. 788 Nicotine Extract
(CL-77)
APPARATUS:
Cigarette, filter paper, funnel, beaker, soap, alcohol lamp or
candle and test tube.
PROCEDURE:
Put some cigarette tobacco into a beaker half full of water. Boil
the contents of the beaker for about ten minutes and then filter,
collecting the clear liquid in the test tube. Place two measures of
soap in a test tube half full of water and shake to dissolve. Pour
the clear nicotine liquid into the soap solution and use as a spray
for plants in the home. This mixture is an infallible insecticide.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook