 The
Science Notebook
Lionel Chem-Lab
- Chapter 27
The
Science Notebook
Lionel Chem-Lab
- Chapter 27
NOTE: This book was published in 1942 as a manual to
accompany several Lionel Chemistry sets of the time. While
some of the experiments and activities here may be safely
done as written, a number of them use chemicals and methods no
longer considered safe. In addition, much of the
information contained in this book about chemistry and other
subjects is outdated and some of it is inaccurate.
Therefore, this book is probably best appreciated for its
historical value rather than as a source for current information
and good experiments. If you try
anything here, please understand that you do so at your
own risk. See our Terms of Use.
Pages 289-317
CHAPTER XXVII
SOME EXPERIMENTS IN CHEMICAL MAGIC
EXPERIMENT No. 789 A Secret Formula For Smoke
(CL-77)
APPARATUS:
Two identical small bottles with wide mouths, hydrochloric acid,
ammonium hydroxide.
PROCEDURE:
In performing this experiment place the bottles four feet apart.
Place two drops of hydrochloric acid in one of the bottles. Shake
the bottle to distribute the solution evenly. Place one drop of
ammonium hydroxide in the other bottle and also shake it. Now
quickly place the bottle containing the ammonium hydroxide on top of
the other bottle (mouth to mouth). Note how quickly the smoke forms
inside the bottles. Remove the top bottle and let some smoke escape.
Note the amount of smoke that has formed inside the bottle.
SUMMARY:
Vapors of hydrochloric acid and ammonium hydroxide react to form
ammonium chloride smoke.
EXPERIMENT No. 790 A Magic White Liquid
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium chloride, sodium carbonate, two glasses and stirring rod.
PROCEDURE:
Dissolve five measures of calcium chloride in a glass one quarter
full of water. Dissolve five measures of sodium carbonate in another
glass one quarter full of water. Pour one solution into the other.
Note the formation of a cream-like substance.
EXPERIMENT No. 791 Imitation Milk
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, sodium thiosulfate, two glasses and a stirring
rod.
PROCEDURE:
Dissolve seven measures of sodium thiosulfate in a glass one quarter
full of water. Dissolve seven measures of sodium bisulfate in
another glass one quarter full of water. Pour one solution into the
other and set aside for several minutes. Note the formation of a
milky precipitate.
EXPERIMENT No. 792 Mysterious Red Writing
(CL-33, CL-44, CL-55, CL-66, CL-77)
289
290
CHEMICAL MAGIC
APPARATUS:
Sodium bicarbonate, phenolphthalein solution, alcohol lamp or
candle, and test tube.
PROCEDURE:
Place three measures of sodium bicarbonate and three drops of
phenolphthalein solution in a test tube containing three or four
drops of water. Stir until the contents form a paste. Dip a clean
pen into the paste, write with it on a piece of paper and allow to
dry. Note the faint pink color. Heat the paper over a flame being
careful not to char the paper. Note the deep, red-colored writing.
EXPERIMENT No. 793 A Mystic Water Glass
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
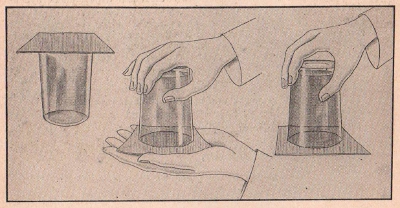
FIGURE 20
APPARATUS:
Cardboard, tumbler.
PROCEDURE:
Place a piece of cardboard tightly over the mouth of a tumbler
filled to the brim with water. Invert the tumbler holding the
cardboard with your hand. Remove your hand. Note that the cardboard
adheres to the mouth of the tumbler so that the water does not
escape.
EXPERIMENT No. 794 Chemical Snow
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Strontium chloride, sodium carbonate, two test tubes, alcohol lamp
or candle.
PROCEDURE:
Dissolve two measures of strontium chloride in a test tube one
quarter filled with water. Dissolve two measures of sodium carbonate
in another test tube one quarter filled with water. Pour this
LIONEL
CHEM-LAB 291
very slowly into the first solution. Note how the particles of the
white precipitate slowly fall to the bottom of the tube.
EXPERIMENT N0. 795 Artificial Icicles
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
A
Hollywood property man makes realistic "icicles" at Warner
Brothers Studios by coating Cellophane with a solution of sodium
silicate or "water glass".
APPARATUS:
Cellophane, sodium silicate, alcohol (drug store), test tubes.
PROCEDURE:
Roll some cellophane between your fingers into the shape of an
icicle and dip it into the sodium silicate vial. Then quickly dip it
into a test tube full of alcohol to harden and note the icicle you
have made. If you want it to appear more realistic, dip the icicle
into some melted paraffin.
EXPERIMENT No. 796 How To Make A Miniature
Snowstorm
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Boric acid, candle or alcohol lamp.
PROCEDURE:
Dissolve twenty measures of boric acid in a test tube half full of
water. Heat if necessary. Note how white flakes fall through the
solution as the boric acid solution cools.
EXPERIMENT No. 797 Chemical Ice
(CL-44, CL-55, CL-66, CL-77)
292
CHEMICAL MAGIC
APPARATUS:
Hydrochloric acid, sodium silicate and test tube.
PROCEDURE:
Pour about one half inch of sodium silicate solution in a test tube
containing four drops of water and shake thoroughly. Add four drops
of hydrochloric acid. Note how quickly the solution freezes.
EXPERIMENT No. 798 A Chemical Chronometer
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium thiosulfate, sodium bisulfate, test tube, four glasses and
stirring rod.
PROCEDURE:
Prepare four sodium thiosulfate solutions as follows: (1) Twelve
measures in a glass half filled with water. (2) Six measures in a
glass half filled with water. (3) Three measures in a glass half
filled with water. (4) Two measures in a glass half filled with
water. Dissolve seven measures of sodium bisulfate in a test tube
filled with water. Add seven drops of this solution to each glass.
Note the different length of time it takes for the milky precipitate
to form in each glass.
EXPERIMENT No. 799 A Magic Egg
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Milk bottle, one large hard boiled egg, piece of absorbent cotton,
alcohol (drug store), a little grease.
PROCEDURE:
Grease the inside of the neck of a milk bottle. Place a peeled hard
boiled egg in the neck of the bottle and try to push it in gently.
Note that the egg will not drop into the bottle. Remove the
egg. Soak a piece of cotton in alcohol, light it and drop it
into the bottle. Replace the egg quickly. Note how easily the
egg now falls into the bottle.
EXPERIMENT No. 800 A Floating Egg
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Two mason jars, salt and an egg.
PROCEDURE:
Fill two jars three quarters full of water. Place four
tablespoonfuls of salt to make a concentrated salt solution in one
of the jars. Place an egg in the first jar and note that it sinks.
Remove the egg from the first jar and put it in the second jar. Note
that it floats. By controlling the strength of the salt solution you
will be able to make the egg float completely, half way or sink.
EXPERIMENT No. 801 An Elastic Egg
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Vinegar (strong) and an egg.
PROCEDURE:
Place an egg in a glass full of vinegar for about twenty-four hours.
Remove the egg, and if it is not soft by this time,
LIONEL
CHEM-LAB 293
repeat the procedure with some fresh vinegar. Note how rubbery the
egg has become.
EXPERIMENT No. 802 Writing With Electricity
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Piece of tinfoil (or a metal pie plate), large nail, two dry cells,
wire, filter paper, starch, sodium iodide solution, candle or
alcohol lamp.
PROCEDURE:
Connect the positive post of one dry cell to the negative post of
the other cell. Connect the nail to the remaining positive terminal
and the tinfoil to the negative terminal. Place a piece of filter
paper over the metal sheet. Dissolve (by heating) one measure of
starch in a test tube half filled with water. Add five drops of
sodium iodide solution. Moisten the filter paper with this solution.
Write with the nail slowly on the filter paper. Note the appearance
of the writing.
EXPERIMENT No. 803 A Magic Tin Can
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Gasoline can with cap, alcohol lamp.
PROCEDURE:
Place a quarter gallon of water in the can. Boil water until all the
air is expelled from the can, then cap it quickly and let cool. Note
what happens to the can.
SUMMARY:
Since the atmospheric pressure is greater than the pressure within
the can, the can collapses.
EXPERIMENT No. 804 Disappearing Flame
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, soft brush, and matches.
PROCEDURE:
Paint a match with sodium silicate solution to a point one quarter
inch away from the head. Light the match. Note how the flame goes
out almost immediately.
EXPERIMENT No. 805 Mysterious Changes
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Congo red paper, blue litmus paper, sodium bisulfate, sodium
carbonate and two glasses.
PROCEDURE:
Dissolve three measures of sodium bisulfate in a glass half filled
with water. Place a strip of congo red paper and a strip of blue
litmus paper in the glass. Note how the litmus paper turns red and
the congo red paper turns dark blue. Dissolve two measures of sodium
carbonate in a glass half filled with water. Place the two test
papers in this. Note how the congo red paper and the blue litmus
paper return to their original colors.
EXPERIMENT No. 806 Making Imitation Blood
(CL-77)
294 CHEMICAL MAGIC
APPARATUS:
Ammonium hydroxide, phenolphthalein solution, three test tubes and
strontium chloride.
PROCEDURE: Dissolve two measures of strontium chloride in a
test tube half full of water. Dampen the hand with some of the
ammonium hydroxide solution and some of the strontium chloride and
allow to dry. Add a little phenolphthalein solution to your hand and
note the red "blood" stain formed. Rinse the hands well upon
completion of the experiment.
EXPERIMENT No. 807 A Chemical Flower Garden
(CL-66, CL-77)
APPARATUS:
Sodium silicate solution, aluminum sulfate, ferrous ammonium
sulfate, ferric chloride, cobalt chloride and a glass.
PROCEDURE:
Mix in a glass one teaspoonful of sodium silicate solution with
three teaspoonfuls of water. Add one measure of aluminum sulfate,
one measure of ferrous ammonium sulfate, one measure of ferric
chloride and one measure of cobalt chloride. Cover the glass and set
aside for a while. Note the fascinating chemical formations.
EXPERIMENT No. 808 A Scientific Thermometer
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, test tube, alcohol lamp or candle.
PROCEDURE:
Dissolve two measures of cobalt chloride in a test tube containing
three or four drops of water. Note the pink color. Heat cautiously
over a flame and note how the color changes to a deep blue. Allow to
cool and note the color change.
SUMMARY:
Heat causes cobalt chloride to lose its color due to the loss of its
water of crystallization. However, when it cools, the original color
is restored because cobalt chloride has the ability to absorb
moisture from the air.
EXPERIMENT No. 809 Magic Serpents
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, sugar, sulfur, tin cover, alcohol lamp or candle.
PROCEDURE:
Mix on a clean sheet of paper one measure of cobalt chloride, one
measure of sulfur and two measures of sugar. Transfer the mixture to
a tin cover and heat it holding the cover away from your body. Note
that in due time the mixture begins to swell up and grows many times
its size.
EXPERIMENT No. 810 A Chemical Smoke
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, heating spoon and alcohol lamp or candle.
LIONEL
CHEM-LAB 295
PROCEDURE:
Place four measures of ammonium chloride in a heating spoon and heat
gently. Note the gradual formation of a thick white smoke.
EXPERIMENT No. 811 Imitation Rubies
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, phenolphthalein solution, glazed paper,
straight pin and stirring rod.
PROCEDURE:
Place a drop of sodium silicate solution on the glazed paper and let
it stand for about a quarter of an hour. Collect a little
phenolphthalein solution on the head of a pin and add it to the drop
of sodium silicate solution. Repeat the operation several times.
Allow to harden overnight. Note the deep red chemical ruby formed by
the action of phenolphthalein on the sodium silicate.
EXPERIMENT No. 812 Synthetic Pearl
(GL-55, CL-66, CL-77)
APPARATUS:
Calcium carbonate, sodium silicate solution, phenolphthalein
solution, heating spoon, wooden bead and straight pin.
PROCEDURE:
Mix two measures of calcium carbonate with two or three drops of
water in the spoon. Add to this ten drops of sodium silicate
solution and mix the ingredients thoroughly. Stick the pin into the
wooden bead. Dip the bead into the mixture repeatedly, allowing the
bead to dry for several minutes after each dipping and after about
five dippings, sprinkle it evenly with calcium carbonate. These
dippings form a layer of hard substance resembling pearl. Repeat
this procedure until the pearl has reached the desired size. Pour a
drop of phenolphthalein solution into the dipping mixture. Dip the
bead into this and note the color.
EXPERIMENT No. 813 A Ghost Picture Comes To Life
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Writing paper, pencil, small soft brush, phenolphthalein solution,
sodium silicate solution.
PROCEDURE:
Divide a sheet of paper in two by drawing a line through the center.
Draw a picture with phenolphthalein solution on one half of the
paper. Brush some sodium silicate solution on the other half. Fold
on the line and bring the divided sections together so that the
phenolphthalein comes into contact with the silicate solution. Note
the appearance of the picture when the paper is opened.
MYSTERIOUS COLOR CHANGES
EXPERIMENT No. 814 Flag Of Victory
(CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, sodium salicylate, ferric chloride, test tubes,
white paper, pencil and soft brush.
296
CHEMICAL MAGIC
PROCEDURE:
Dissolve the following chemicals in separate test tubes half full of
water. (1) One measure of sodium ferrocyanide. (2) One measure of
sodium salicylate. (3) One measure of ferric chloride. Draw the
outline of an American Flag on a sheet of white paper. Paint the
part of the Hag which is to be blue with the sodium ferrocyanide
solution and the stripes which are to be red with the sodium
salicylate. Allow to dry. Paint the whole surface of the flag with
the ferric chloride solution.
EXPERIMENT No. 815 A Mysterious Color
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Starch, ferric ammonium sulfate, sodium bisulfate, sodium iodide
solution, two test tubes and alcohol lamp or candle.
PROCEDURE:
Prepare some starch test solution as described in Experiment No.
688. Pour a test tube full of warm water into another test tube
containing one measure of starch and a few drops of water. Pour a
few drops of this in-to the cold starch solution and note the
resulting deep blue color. Heat this preparation to boiling and note
the disappearance of the blue color. Allow to cool and then note the
reappearance of the characteristic blue color.
EXPERIMENT No. 816 Colored Wonders
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, cobalt chloride, strontium chloride,
ferric ammonium sulfate, phenolphthalein solution and candle or
alcohol lamp.
PROCEDURE:
Place one half inch of sodium silicate solution in a test tube one
third filled with water. Dissolve two measures of cobalt chloride
into another test tube one quarter filled with water. Pour a few
drops of the contents of both test tubes into a third test tube, the
total amount not exceeding more than one half inch. Pour the sodium silicate solution
first. Note the formation of a blue precipitate. Dissolve
one measure of strontium chloride in a test tube one quarter filled
with water. Put some of this, together with an equal amount of
sodium silicate solution, into the test tube containing the blue
precipitate. Note, this time, the formation of a white precipitate.
Heat one measure of ferric ammonium sulfate in a test tube one
quarter filled with water. Pour some of this with an equal amount of
sodium silicate solution into the color test tube. Note the
formation of a brown precipitate. Dissolve one measure of strontium
chloride in a test tube one quarter filled with water. Add two drops
of phenolphthalein solution. Pour a few drops of this solution with
an equal amount of sodium silicate solution into the color test
tube. Note the formation of a red precipitate.
EXPERIMENT No. 817 A Vanishing Blue Color
(CL-66, CL-77)
APPARATUS:
Tincture of iodine, starch, mortar and pestle, milk bottle.
LIONEL
CHEM-LAB 297
PROCEDURE:
Boil a quart of water in a pan. Place fifteen measures of starch in
the mortar and add a few drops of water to make a paste. Pour some
boiling water in the paste and pour the paste solution into the pan.
Allow this to boil for several minutes, then set aside to cool. Pour
the cooled starch solution into a milk bottle, adding enough
tincture of iodine to turn solution blue. Pour the liquid back into
the pan and boil again. Note how the liquid loses its color.
EXPERIMENT No. 818 Grenadine And Water From The
Same Glass
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, phenolphthalein solution, sodium carbonate, two
glasses and eye dropper.
PROCEDURE:
Dissolve four measures of sodium bisulfate in a glass filled with
water. Add three drops of phenolphthalein solution. Place three
measures of sodium carbonate in the other glass. Add to this a
sufficient amount of water to dissolve the chemicals. Pour the
solution of the first glass into the second. Note the gradual
disappearance of the initial red color.
EXPERIMENT No. 819 A Mysterious Pitcher Of Bluing
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric ammonium sulfate, sodium carbonate,
opaque pitcher and five glasses.
PROCEDURE:
Place the following chemicals in the three glasses together with a
few drops of water. Glass No. 1, one measure of sodium ferrocyanide.
Glass No. 2, one measure of ferric ammonium sulfate. Glass No. 3,
seven measures of sodium carbonate. Pour water from the pitcher into
the three glasses, then empty the contents of glasses No. 1 and No.
2 back into the pitcher. Pour the contents of the pitcher into the
unused glasses. Note the blue color. Pour the solutions back into
the pitcher, this time together with the contents of glass No. 3.
Fill the glasses once more. Note that the liquid has lost its color.
EXPERIMENT No. 820 Water Transformed Into
Grenadine
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Large opaque pitcher, three glasses, sodium carbonate,
phenolphthalein solution, sodium bisulfate.
PROCEDURE:
Place the following chemicals in the glasses together with a few
drops of water. Glass No. 1, one measure of sodium carbonate. Glass
No. 2, three drops of phenolphthalein solution. Glass No. 3, five
measures of sodium bisulfate. Pour water from the pitcher into Glass
No. 1 and glass No. 2 but not glass No. 3. Pour the contents of the
pitcher into the unused glasses and note the wine color. Empty the
glasses back into the pitcher together with the sodium
298
CHEMICAL MAGIC
bisulfate solution and then return the contents to the glasses. Note
that the liquid has lost its color.
EXPERIMENT No. 821 Red, Blue, And Gold From One
Glass
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, sodium bisulfate, sodium salicylate, ferric
ammonium sulfate, four glasses and stirring rod.
PROCEDURE:
Fill each glass halfway with water. Dissolve the following chemicals
in the glasses by stirring: Glass No. 1, half measure of sodium
ferrocyanide. Glass No. 2, five measures of sodium bisulfate. Glass
No. 3, one measure of sodium salicylate. Glass No. 4, four measures
of ferric ammonium sulfate. Pour a little of the contents of glass
No. 4 into each of the other three glasses. Note the blue, yellow
and red colors.
EXPERIMENT No. 822 Changing Water To Colors Of
Red And White
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, phenolphthalein solution, sodium bisulfate, two
glasses, eye dropper and stirring rod.
PROCEDURE:
Put three measures of sodium carbonate in a glass half full of water
and stir to dissolve. Add three drops of phenolphthalein solution.
Note the resulting red color. Put two measures of sodium bisulfate
in another glass together with four drops of water and stir to
dissolve. Pour the solution of the first glass into the other. Note
the change in color.
SUMMARY:
Phenolphthalein forms a red color with a basic salt (sodium
carbonate), and a colorless solution in the presence of a strong
acid salt.
EXPERIMENT No. 823 Many Different Colors From One
Pitcher
(CL-66, 0L-77)
APPARATUS:
Ferric ammonium sulfate, tannic acid, strontium chloride, sodium
carbonate, sodium salicylate, cobalt chloride, calcium oxide,
phenolphthalein solution, sodium ferrocyanide, mixed dyes, pitcher
and glasses.
PROCEDURE:
Place in: Glass No. 1, one measure of ferric ammonium sulfate and
one measure of tannic acid. Glass No. 2, three measures of strontium
chloride and three measures of sodium carbonate. Glass No. 3, a half
measure of sodium salicylate and half measure of ferric ammonium
sulfate. Glass No. 4, three measures of cobalt chloride and two
measures of sodium carbonate. Glass No. 5, one measure of calcium
oxide and two drops of phenolphthalein solution. Glass No. 6, one
measure of ferric ammonium sulfate and four measures of sodium
carbonate. Glass No. 7, one third measure of sodium ferrocyanide and
one half measure of ferric ammonium sulfate. Glass No. 8, two
measures, of cobalt chloride and one measure
LIONEL
CHEM-LAB 299
of sodium ferrocyanide. Glass No. 9, one eighth measure of mixed
dyes. Fill each glass with water and stir. Note the different color
in each glass.
EXPERIMENT No. 824 Chemical Rainbow Streamers
(CL-66, CL-77)
APPARATUS:
Milk bottle, mixed dyes.
PROCEDURE:
Fill the milk bottle with water and set aside for several minutes
until the water becomes perfectly still. Place one measure of mixed
dyes on the surface of the water. Note the striking color effect.
EXPERIMENT No. 825 Colors In Motion
(CL-66, CL-77)
APPARATUS:
Sodium carbonate, mixed dyes, aluminum sulfate, stirring rod and a
glass.
PROCEDURE:
Dissolve two measures of sodium carbonate in a glass filled with
water. Allow the water in the glass to become perfectly still. Add
one measure of mixed dyes and note the colored streamers sinking to
the bottom. Drop into this two measures of aluminum sulfate when the
dye streamers have reached the bottom and note the formation of a
beautifully colored precipitate. Observe the rising of the
precipitate to the top of the liquid.
EXPERIMENT No. 826 A Vanishing Color
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, phenolphthalein solution, milk bottle, glass,
vinegar and baking soda.
PROCEDURE:
Dissolve one half measure of sodium carbonate in the milk bottle
half full of water. Add to this two drops of phenolphthalein
solution. Place a teaspoonful of baking soda into a glass and add
four drops of vinegar. Note the effervescence. Hold the glass near
the mouth of the milk bottle for several minutes without permitting
any of the contents of the glass to fall into the bottle. Cover the
bottle and set aside for several hours. Note in due time how the
color in the bottle disappears.
EXPERIMENT No. 827 Crimson Fire
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Strontium chloride, potassium nitrate, sulfur, powdered charcoal.
PROCEDURE:
Perform this experiment outdoors. Mix thoroughly on a clean sheet of
paper two measures of potassium nitrate, one measure of strontium
chloride, one measure of sulfur and two measures of powdered
charcoal. Place this mixture in an iron vessel and from a safe
distance, ignite it with a match. Keep your face well away from the
reaction. Note the color of the flame.
300
CHEMICAL MAGIC
EXPERIMENT No. 828 Reproducing Different Colors
With Soda Pop
(GL-77)
APPARATUS:
Benedict’s solution, test tubes, cream flavored soda pop, alcohol
lamp or candle.
PROCEDURE:
Place three drops of Benedict’s solution into a test tube half full
of cream soda. Note color of soda. Boil solution for a few minutes
and note all the color changes. Benedict’s solution imparts a green
color to the soda which is converted to an orange color by heating.
EXPERIMENT No. 829 An Array Or Chemical Colors
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, sodium salicylate, sodium ferrocyanide,
sodium silicate solution, phenolphthalein solution, five glasses and
a stirring rod.
PROCEDURE:
Place the following chemicals in five different glasses. Glass No.
1, one measure of ferric ammonium sulfate. Glass No. 2, one measure
of sodium salicylate. Glass No. 3, one measure of sodium
ferrocyanide. Glass No. 4, one spoonful of sodium silicate solution.
Glass No. 5, three drops of phenolphthalein solution. Pour water
into the first glass and stir to dissolve. Pour this into the second
glass, and the second into the third, the third into the fourth, and
the fourth into the fifth, stirring each time. Note the variety of
colors produced after each process.
EXPERIMENT No. 830 Coloring Water Red, White And
Blue
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium salicylate, strontium chloride, sodium ferrocyanide, ferric
ammonium sulfate and four glasses.
PROCEDURE:
Fill three glasses half full of water. Place one measure of sodium
salicylate in the first glass, four measures of strontium chloride
in the second, two measures of sodium ferrocyanide in the third and
six measures of ferric ammonium sulfate into the fourth glass. Stir
to dissolve. Pour some of the contents of the fourth glass into the
other three glasses and stir. Note the red, white and blue colors.
EXPERIMENT No. 831 How To Make Colored Bubbles
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Phenolphthalein solution, sodium carbonate, tartaric acid, sodium
ferrocyanide, test tube and ferric ammonium sulfate.
PROCEDURE:
Dissolve one measure of sodium carbonate and one drop of
phenolphthalein solution in a test tube one half full of water. Note
the red color of the solution. Add one measure of tartaric acid and
note the bubbling reaction and the disappearance of the color. Add
two measures of sodium carbonate. Note the bubbling reaction and the
return of the original color. Add one measure of sodium
LIONEL
CHEM-LAB 301
ferrocyanide, one measure of ferric ammonium sulfate and two
measures of tartaric acid to the contents of the test tube. Note the
blue bubbles which form on the surface of the solution.
MAGIC METALS
EXPERIMENT No. 832 A Magic Penny
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, test tubes, mercurochrome, (drug store), small
glass, bright penny and steel wool.
PROCEDURE:
Fill a test tube about one quarter full of mercurochrome adding an
equal amount of water and two measures of sodium bisulfate. Heat to
boiling for a few minutes. Pour this solution into a small glass.
Drop a bright penny into the solution. Note that within a few
moments the coin becomes silvery.
EXPERIMENT No. 833 Bronze Particles From Iron
Filings
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, powdered iron metal and test tube.
PROCEDURE:
Dissolve three measures of copper sulfate in a test tube half filled
with water, then add two measures of powdered iron. Shake the test
tube for a few moments. Let the powdered iron settle to the bottom
of the test tube, then pour off the liquid. Note the copper coating
on the iron.
EXPERIMENT No. 834 Making A Penny From A Dime
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, test tube, glass, small iron nail and a dime.
PROCEDURE:
Dissolve two measures of copper sulfate in a test tube half full of
water. Place a dime and an iron nail in the sulfate solution in such
a way that they do not touch each other. Note the gradual formation
of a red copper coating on the coin.
EXPERIMENT No. 835 Transforming Steel Into Copper
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, knife and glass.
PROCEDURE:
Dissolve ten measures of copper sulfate in a glass half full of
water. Place the blade of the knife in this solution for an hour.
Remove the knife. Note the red copper coating.
SECRET FORMULAS FOR INKS
EXPERIMENT No. 836 Disappearing Red Writing
(CL-77)
302
CHEMICAL MAGIC
APPARATUS:
Phenolphthalein solution, ammonium hydroxide, white paper and soft
brush.
PROCEDURE:
Dip the brush in a solution of ammonium hydroxide and write with it
on a clean piece of paper. Clean the brush. Allow the writing to
dry, then dip the brush into phenolphthalein solution and retrace
the writing. Note that as the writing dries the red color
disappears.
EXPERIMENT No. 837 Disappearing Blue Writing
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, test tube, white paper, pen and alcohol lamp.
PROCEDURE:
Dissolve four measures of cobalt chloride in a test tube half full
of water. Write with this on the paper and allow to dry. Heat the
paper gently near the alcohol flame. Note the appearance of the blue
colored writing. Hold the paper over some boiling water and note how
the writing disappears because cobalt chloride is colorless when
moist.
EXPERIMENT No. 838 A Magic Tannate
(CL-66, CL-77)
APPARATUS:
Ferric chloride, tannic acid, calcium hypochlorite, small brush,
sheet of white paper, tartaric acid, test tubes.
PROCEDURE:
Dissolve two measures of ferric chloride in a test tube half full of
water. Dip the brush into the solution and write a few lines on the
white paper. Allow to dry thoroughly. Dissolve one measure of tannic
acid in another test tube half full of water. Wet the writing with
this solution and note the blue writing. Dissolve one measure of
calcium hypochlorite and a half measure of tartaric acid in another
test tube half full of water. Dip the brush into this solution and
retrace the blue writing. Note how the writing gradually
disappears.
EXPERIMENT No. 839 How To Make Dark Red
Sympathetic Ink
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium salicylate, ferric ammonium sulfate, two test tubes, pen,
sheet of white paper and a wad of cotton.
PROCEDURE:
Dissolve one measure of sodium salicylate into a test tube half
filled with water. Dip the pen into the solution and write with it.
Allow to dry. Dissolve two measures of ferric ammonium sulfate in
another test tube one fourth filled with water. Spread this over the
writing with the cotton. Note the appearance of red writing.
EXPERIMENT No. 840 Onion Sympathetic Ink
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Pen, sheet of white paper, onion juice, alcohol lamp.
LIONEL
CHEM-LAB 303
PROCEDURE:
Dip the clean pen in the onion juice and write with it on a sheet of
paper. Hold the paper over the alcohol lamp. Note the appearance of
the writing.
EXPERIMENT No. 841 Making Light Red Ink
(CL-55, CL-66, GL-77)
APPARATUS:
Sodium ferrocyanide, copper sulfate, sheet of white paper, pen,
piece of cotton and two test tubes.
PROCEDURE:
Dissolve two measures of sodium ferrocyanide in a test tube one
fourth filled with water. Write with this on the paper and allow to
dry. Put two measures of copper sulfate in another test tube one
fourth filled with water and shake to dissolve. Paint this solution
over the writing with a piece of cotton. Note the appearance of the
red writing.
EXPERIMENT No. 842 Making Sympathetic Ink
From Iron
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, test tube, alcohol lamp or candle, pen and
white paper.
PROCEDURE:
Dissolve two measures of ferric ammonium sulfate in a test tube one
fourth filled with water. Write with this on a sheet of paper and
allow to dry. Heat the paper near the alcohol flame. Note the
appearance of the writing.
EXPERIMENT No. 843 Making Writing Disappear
(CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric chloride, sodium ferrocyanide, test tube, pen, sheet of white
paper and sodium carbonate.
PROCEDURE:
Dissolve one measure of ferric chloride and an equal amount of
sodium ferrocyanide in a test tube half full of water. Dip the pen
into this solution and write with it on the paper. Dissolve two
measures of sodium carbonate in another test tube one fourth filled
with water. Dip the pen into this and trace over the writing. Note
how the writing disappears.
EXPERIMENT No. 844 Making Dark Green Sympathetic
Ink
(CL-55, CL-66, CL-77)
APPARATUS:
Copper sulfate, ammonium chloride, candle or alcohol lamp, pen,
sheet of white paper and test tube.
PROCEDURE:
Dissolve by heating two measures of copper sulfate and one measure
of ammonium chloride in a test tube one fourth full of water. Write
with this and allow to dry. Heat the paper near a flame. Note the
appearance of a dark, olive-green writing.
EXPERIMENT No. 845 Making Ink From Grapefruit
(CL-1), CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
304
CHEMICAL MAGIC
APPARATUS:
Grapefruit juice, piece of paper, pen and alcohol lamp.
PROCEDURE:
Dip a pen into some grapefruit juice and write with it on a sheet of
paper. Hold the paper near the alcohol flame. Note the appearance of
the writing.
EXPERIMENT No. 846 Blue Sympathetic Ink
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, copper sulfate, sodium bisulfate, two test tubes,
pen, sheet of white paper and a soft brush.
PROCEDURE:
Dissolve two measures of copper sulfate and three measures of sodium
bisulfate in a test tube one quarter full of water. Dip a clean pen
into this solution, write with it and allow to dry. Dissolve one
measure of sodium carbonate in a test tube one fourth full of water.
Paint the writing with this solution. Note that the writing becomes
blue.
EXPERIMENT No. 847 Yellow Sympathetiic Ink
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, test tube, clean pen, candle or alcohol lamp.
PROCEDURE:
Place five measures of ammonium chloride in a test tube half filled
with water and shake to dissolve. Dip the pen in the solution, write
with it and allow to dry. Hold the paper near the lamp or candle.
Note that the writing gradually becomes yellow.
EXPERIMENT No. 848 Sympathetic Ink From Vinegar
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Vinegar, pen, sheet of paper, alcohol lamp or candle.
PROCEDURE:
Dip the pen into the vinegar, write with it on the paper and allow
it to dry. Heat the paper near the alcohol lamp or candle. Note how
the writing appears.
EXPERIMENT No. 849 Rust Sympathetic Ink
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, sodium carbonate, two test tubes, pen,
sheet of white paper and a wad of cotton.
PROCEDURE:
Dissolve four measures of ferric ammonium sulfate in a test tube
half filled with water. Dip pen in this solution and write with it
on the paper. Dissolve four measures of sodium carbonate in another
test tube one fourth filled with water. Swab this solution over the
writing with the cotton.
EXPERIMENT No. 850 Developing Blue Sympathetic
Ink
(CL-55. CL-66, CL-77)
LIONEL
CHEM-LAB 305
APPARATUS:
Copper sulfate, ammonium hydroxide (household ammonia), pen, sheet
of paper and soft brush.
PROCEDURE:
Dissolve two measures of copper sulfate in a test tube one fourth
filled with water. Using a clean pen, write with this and allow to
dry. Paint over the writing with a brush moistened with some
ammonium hydroxide. Note the deep blue writing.
CHEMICAL WITCHCRAFT
EXPERIMENT No. 851 A Mystic Cloth
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Cobalt chloride, small piece of cloth and test tube.
PROCEDURE:
Dissolve three measures of cobalt chloride in a test tube three
quarters full of water. Soak the cloth in this solution, then remove
and hold it near a heater to dry completely. Note the blue color.
Squeeze the dry cloth in your hands and blow through it for several
minutes. Note how the cloth becomes colorless. Reheat the cloth and
note that the blue color of the cloth is restored.
EXPERIMENT No. 852 Artificial Growth
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sugar, sulfur, cobalt chloride, heating spoon, candle or alcohol
lamp.
PROCEDURE:
Put four measures of sugar, two measures of sulfur, and two measures
of cobalt chloride in the heating spoon. Heat the mixture. Note that
in due time, the mixture increases in size.
EXPERIMENT N0. 853 A Green Flame
(CL-77)
APPARATUS:
Boric acid, alcohol, test tube and heating spoon.
PROCEDURE:
Dissolve two measures of boric acid in a test tube one third full of
alcohol. Pour a little of this on the spoon and ignite it. Note how
the alcohol burns with a green flame proving that a borate is
present.
EXPERIMENT No. 854 Bouncing Bubbles
(CL-55, CL-66, CL-77)
APPARATUS:
Soap shavings, glycerine, large glass and bubble pipe (store).
PROCEDURE:
Mix two teaspoonfuls of soap shavings and one half teaspoonful of
glycerine in a glass of warm water. Transfer the mixture to a large
glass and stir it to form some suds. Immerse the bowl of the pipe in
this solution, then blow through the pipe gently. Note the bubbles.
Allow some of the bubbles to float down upon a soft surface. Note
how they bounce without breaking.
306
CHEMICAL MAGIC
EXPERIMENT No. 855 Making A Red Solution White
(CL-33, CL-44, CL-55, CL-66,, CL-77)
APPARATUS:
Calcium oxide, phenolphthalein, two glasses and glass tubing.
PROCEDURE:
Dissolve two spoonfuls of sugar in a glass of water. Add to this
three measures of calcium oxide and stir for a minute or two. Set
aside until the precipitate settles at the bottom. Pour off some of
the clear solution into another glass and add three drops of
phenolphthalein solution. Note the red color. Blow into the red
liquid through the glass tubing for several minutes. Note how the
solution gradually becomes white.
EXPERIMENT No. 856 A Bouncing Mothball
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Vinegar, sodium carbonate, mothball and small bottle.
PROCEDURE:
Place eight measures of sodium carbonate and a mothball in a small
bottle half full of vinegar. Note how it rises and sinks at regular
intervals.
EXPERIMENT No. 857 Making Elastic Bones
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Vinegar and wish bone of chicken.
PROCEDURE:
Clean the bone thoroughly and place it in strong vinegar for one
day. Remove the bone from the vinegar. Note how the bone will bend
without breaking.
CHEMICAL ODORS
EXPERIMENT No. 858 A Secret Formula For Smelling
Salts
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ammonium chloride, calcium oxide, test tube and candle or alcohol
lamp.
PROCEDURE:
Put two measures of ammonium chloride in a test tube. Add to this
two measures of calcium oxide and heat for a few minutes. Note the
odor of the gas given off.
EXPERIMENT No. 859 A Pungent Odor
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfite, tartaric acid and a test tube.
PROCEDURE:
Put three measures of sodium bisulfite in a test tube. Add to this
three measures of tartaric acid and a few drops of water. Note
the strong odor.
EXPERIMENT No. 860 A Formula For Violet Perfume
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
LIONEL
CHEM-LAB 307
APPARATUS:
Sodium carbonate, castor oil, alcohol lamp or candle and test tubes.
PROCEDURE:
Place one measure of sodium carbonate and four drops of castor oil
in a test tube. Heat the test tube until a white vapor rises. Allow
the tube to cool. Smell the contents cautiously and note the odor of
violet.
EXPERIMENT No. 861 An Offensive Odor
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sulfur, piece of candle, test tube, alcohol lamp or candle.
PROCEDURE:
Put three measures of sulfur in a test tube together with some
candle chips. Heat this material. Note the obnoxious odor which
emanates from the test tube.
EXPERIMENT No. 862 Tear Gas
(CL-55, CL-66, CL-77)
APPARATUS:
Glycerine, sodium bisulfate, alcohol lamp or candle and test tube.
PROCEDURE:
Place one drop of sodium bisulfate in a test tube containing two
drops of glycerine. Heat the test tube gently. Stop heating as soon
as vapor begins to rise from the test tube.
CAUTION:
This gas will irritate the eyes and the membrane of the nose.
Therefore, care should he taken not to inhale too much of it.
EXPERIMENT No. 863 The Odor Of Rotten Eggs
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Hydrochloric acid, iron sulfide, test tube.
PROCEDURE:
Place two measures of iron sulfide in a test tube. To this add Hve
drops of hydrochloric acid. Note the disagreeable odor of rotten
eggs.
CAUTION:
As soon as the odor is perceptible, destroy the solution by flushing
the test tube with running water or the house will be filled with
this obnoxious odor.
MAGIC INKS AND PAPERS
EXPERIMENT No. 864 "Striped" Paint
(CL-33, CL-44, OL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, sodium salicylate, tannic acid, ferric
chloride, soft brush, three test tubes and one piece of paper.
PROCEDURE:
Dissolve the following chemicals in separate test tubes half full of water: one
measure of ferrocyanide, one measure of sodium salicylate, and one
measure of tannic acid. Paint each solution
308
CHEMICAL MAGIC
in stripes on the paper making certain that the stripes touch each
other. Allow this to dry thoroughly. Dissolve two measures of ferric
chloride in a fourth test tube half full of water. Clean the brush,
dip it into this solution and paint over the stripes. Note the
different colored stripes formed.
EXPERIMENT No. 865 An Ink In Three Different
Colors
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, sodium salicylate, tannic acid, ferric ammonium
sulfate, three pieces of white paper, soft brush, pen and four test
tubes.
PROCEDURE:
Dissolve the following chemicals in separate test tubes half filled
with water: one measure of sodium ferrocyanide, one measure of
sodium salicylate, and one measure of tannic acid. Paint the
solutions with a brush on the three pieces of paper using one
solution for each paper and cleaning the brush thoroughly after each
application. Set these aside to dry. Dissolve two measures of ferric
ammonium sulfate in a fourth test tube half filled with water. Dip
the pen into this solution and write with it on the three pieces of
paper. Note the three different colors.
EXPERIMENT No. 866 An Ink In Four Different
Colors
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, tannic acid, ferric ammonium sulfate, sodium
salicylate, sodium chromate, soft brush, four pieces of white paper
and test tubes.
PROCEDURE:
Dissolve the following chemicals in separate test tubes one quarter
full of water: one measure of tannic acid, one measure of sodium
ferrocyanide, one measure of sodium chromate, and one measure of
sodium salicylate. Paint the solution with a brush on the four
pieces of paper using one solution for each paper and cleaning the
brush thoroughly after each application. Set aside to dry and in the
meantime dissolve two measures of ferric ammonium sulfate in a test
tube one quarter full of water. Dip the brush into this solution and
write on the four pieces of paper. Note the different colors as the
ferric ammonium sulfate is painted over each sheet of paper.
EXPERIMENT No. 867 Red Writing Miracle Ink
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sheet of white paper, sodium salicylate, ferric ammonium sulfate and
pen.
PROCEDURE:
Place a measure of sodium salicylate and a measure of ferric
ammonium sulfate on the paper mixing the chemicals together. Rub
this over the paper with another piece of paper and shake off the
excess. Dip the pen point in water and write with it on the paper.
Note the red writing.
LIONEL
CHEM-LAB 309
EXPERIMENT N0. 868 Writing With Salt
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Table salt, sheet of white paper, pen, soft lead pencil and test
tube.
PROCEDURE:
Dissolve seven measures of salt in a test tube half filled with
Water. Write with this solution on the paper and allow to dry. Rub
over the writing with the pencil. Note how the writing begins to
appear.
EXPERIMENT No. 869 Water Writing
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Broad pen point, thin ink, blotter, white stationery.
PROCEDURE:
Write a few words on the paper with pen and water, making the
letters large. Allow to dry. Paint this with some thin ink. Blot the
ink immediately. Note how the writing stands out.
EXPERIMENT No. 870 Making Blue Writing With
Iodine
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, sodium bisulfate, sodium iodide solution,
starched piece of cloth and test tube.
PROCEDURE:
Place a measure of ferric ammonium sulfate and an equal amount of
sodium bisulfate in a test tube half filled with water. Add to this
a few drops of sodium iodide solution shaking the tube well. Note
the brown color of the solution. Write with this on the starched
cloth. Note that the color of the writing turns from blue to brown.
EXPERIMENT N0. 871 Many Color Writings
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tannic acid, sodium ferrocyanide, sodium salicylate, ferric ammonium
sulfate, pen, sheet of paper, four test tubes.
PROCEDURE:
Dissolve the following chemicals in separate test tubes one fourth
filled with water: one measure of tannic acid, one measure of sodium
salicylate, and one measure of sodium ferrocyanide. Draw some sort
of design with the solutions by crossing the lines of one solution
with the lines of the other. Clean the pen before the application of
each solution. Dissolve one measure of ferric ammonium sulfate in a
fourth test tube one fourth filled with water. Write with
this along the lines made by the other three solutions. Note the
striking color effects.
EXPERIMENT No. 872 Scientific Blue Writing
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric ammonium sulfate, piece of white paper
and pen.
310
CHEMICAL MAGIC
PROCEDURE:
Place a measure of sodium ferrocyanide and a measure of ferric
ammonium sulfate on the paper mixing them together. Rub this gently
over the surface of the paper and shake off the excess. Dip the pen
point in water and write with it on the paper. Note the blue
writing.
EXPERIMENT No. 873 How To Make Black Writing
Appear
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tannic acid, ferric ammonium sulfate, two test tubes, alcohol lamp
or candle, a sheet of paper and a pen.
PROCEDURE:
Place two measures of tannic acid in a test tube half full of water
and heat for a few minutes to dissolve. Dip the pen in this solution
and write on a sheet of paper. Dissolve one measure of ferric
ammonium sulfate in another test tube one fourth full of water. Dip
a clean pen into this solution and write along the lines made by the
tannic acid solution. Note the appearance of the black writing.
EXPERIMENT No. 874 Making Red Writing Appear
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium salicylate, ferric ammonium sulfate, test tube, pen and a
sheet of white paper.
PROCEDURE:
Dissolve one measure of sodium salicylate in a test tube one fourth
full of water. Dip a clean pen into the solution and write on the
paper. Dissolve one measure of ferric ammonium sulfate in another
test tube one fourth full of water. Dip a clean pen into this
solution and trace out the writing made by the sodium salicylate
solution. Note the appearance of red writing.
EXPERIMENT No. 875 Blue Writing Appears When
Traced With A Pen
(CL-11, CL-22, CL-33, CL-44, CL-65, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric chloride, one sheet of paper, pen and
two test tubes.
PROCEDURE:
Dissolve one measure of sodium ferrocyanide in a test tube one
fourth full of water. Dip the clean pen into this solution and write
on the paper. Dissolve one measure of ferric ammonium sulfate in
another test tube one fourth full of water. Dip a clean pen into
this and trace the lines of the writing made by the sodium
ferrocyanide solution. Note the appearance of blue writing.
EXPERIMENT No. 876 How To Make Writing Appear
When Blotted
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium salicylate, ferric ammonium sulfate, piece of writing paper,
pen, blotting paper and two test tubes.
PROCEDURE:
Prepare a solution of two measures of sodium salicylate in a test
tube half filled with water. Write with this on the paper.
LIONEL
CHEM-LAB 311
Allow to dry. Dissolve two measures of ferric ammonium sulfate in
another test tube half filled with water. Moisten the blotting paper
with this solution. Blot the writing with the blotting paper, Note
the appearance of the red writing.
EXPERIMENT No. 877 Preparing A Mistake Black
Blotter
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tannic acid, ferric ammonium sulfate, white blotting paper, candle
or alcohol lamp, white paper, pen and two test tubes.
PROCEDURE:
Dissolve by heating two measures of tannic acid in a test tube half
filled with water. Write with this on the paper. Allow to dry.
Dissolve two measures of ferric ammonium sulfate into another test
tube half filled with water. Moisten in this a piece of blotting
paper. Pass the blotting paper over the writing. Note how the
writing appears black.
EXPERIMENT No. 878 Making A Mystic Red Blotter
(CL-77)
APPARATUS:
Ammonium hydroxide, phenolphthalein solution, blotter, writing paper
and pen.
PROCEDURE:
Dip the pen into a solution of phenolphthalein solution and write a
few words. Moisten a blotter with a few drops of ammonium hydroxide
and place the moistened part on the writing. Note the red writing
which appears on the paper.
EXPERIMENT No. 879 Making A Mystic Blue Blotter
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric ammonium sulfate, sheet of writing
paper, pen, blotter and two test tubes.
PROCEDURE:
Dissolve two measures of sodium ferrocyanide in a test tube half
full of water. Write with this on a sheet of paper. Allow to dry.
Prepare a solution of two measures of ferric ammonium sulfate in a
test tube half full of water. Moisten the blotter with this solution
and place it over the writing. Note the blue writing which appears
on the paper.
EXPERIMENT No. 880 A Magic Black Writing Paper
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sheet of white paper, tannic acid, ferric ammonium sulfate and pen
point.
PROCEDURE:
Place a measure of tannic acid and a measure of ferric ammonium
sulfate on the paper mixing the chemicals together. Rub the mixture
with a piece of paper over the surface of the writing paper. Remove
the excess mixture and write on the paper with water. Note the black
writing.
312
CHEMICAL MAGIC
EXPERIMENT No. 881 White Writing On Blue Prints
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, ferric chloride, sheet of white paper, sodium
carbonate, test tube, calcium oxide, pen and soft brush.
PROCEDURE:
Place two measures of sodium ferrocyanide and two measures of ferric
chloride in a test tube one quarter full of water. Shake test tube
until the mixture dissolves. Dip the soft brush into this solution
and paint a wide stripe on the white paper. Allow to dry. In the
meantime prepare a solution of sodium hydroxide as described in
Experiment No. 344. Dip the brush into this solution and write on
the blue stripe. Note the white writing formed when sodium hydroxide
solution was painted on the blue paper.
EXPERIMENT No. 882 Red Writing On Blue Prints
(CL-66. CL-77)
APPARATUS:
Sodium ferrocyanide, ferric chloride, sheet of white paper, sodium
carbonate, calcium oxide, phenolphthalein solution, test tubes, soft
brush.
PROCEDURE:
Dissolve two measures of sodium ferrocyanide and two measures of
ferric chloride in a test tube one quarter full of water. Dip a
brush into this solution and paint a wide stripe on the white paper.
Allow it to dry. Meantime, prepare a solution of sodium hydroxide as
described in Experiment No. 344. Dip the brush into this solution
and write on the blue stripe. Note the white writing formed when the
sodium hydroxide solution was painted on the blue paper. Retrace the
white writing with phenolphthalein solution. Note how
phenolphthalein is able to convert the writing to a red color.
EXPERIMENT No. 883 Preparing A Magic White Ink
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfite, tartaric acid, colored paper, pen and test tube.
PROCEDURE:
Dissolve three measures of sodium bisulfite and three measures of
tartaric acid in a test tube one half full of water. Write with this
solution on the colored paper. Note the white writing on the colored
paper.
EXPERIMENT No. 884 White Writing On Black Paper
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium bisulfate, sodium carbonate, two test tubes, sheet of paper
and alcohol lamp or candle.
PROCEDURE:
Dissolve seven measures of sodium bisulfate in a test tube one third
full of water. Dip into this a piece of white paper and allow it to
dry. Dissolve two measures of sodium carbonate in another test tube
one fourth full of water. Write with this on the paper. Heat the
paper near the flame until it becomes scorched with-
LIONEL
CHEM-LAB 313
out setting the paper on fire. Note the appearance of the white
writing.
CONJURING COLORS
EXPERIMENT No. 885 Water to "Blood” And "Stone”
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium silicate solution, phenolphthalein solution, aluminum
sulfate, three test tubes and glass.
PROCEDURE:
Place about one inch of sodium silicate solution in a test tube.
Fill the tube half way with water. Place three drops of
phenolphthalein solution in a second test tube one third filled with
water. Dissolve five measures of aluminum sulfate in a third test
tube one third filled with water. Hold the three tubes in one hand
and pour the contents simultaneously into the glass. Note the
formation of a red liquid and its subsequent change to a red solid.
EXPERIMENT No. 886 Changing Waterr To "W1ne"
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tincture of iodine (drug store) and a glass.
PROCEDURE:
Pour five drops of tincture of iodine into a glass and set it aside
until the liquid dries. Pour water into the glass until it is half
full. Note the red color formation.
EXPERIMENT No. 887 Changing Water to "Wine"
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Phenolphthalein solution, sodium carbonate, calcium oxide, tartaric
acid, stirring rod.
PROCEDURE:
Fill a glass half full of water adding three drops of
phenolphthalein solution. Add one half measure of sodium carbonate
and one half measure of calcium oxide. Note the wine color. Add to
this solution two measures of tartaric acid. Note how the solution
becomes colorless. Add to the solution four measures of sodium
carbonate and two measures of calcium oxide. Note how the red color
returns.
SUMMARY:
Phenolphthalein in the presence of an alkali, formed in the reaction
between calcium oxide and sodium carbonate, turns the solution red.
The solution becomes colorless when tartaric acid is added because
neutralization occurs. The addition of calcium oxide and sodium
carbonate causes the solution to be basic, thus the solution becomes
red again.
EXPERIMENT No. 888 Changing Wine To Water
(CL-83. CL-44, CL-55, CL-66, CL-77)
314
CHEMICAL MAGIC
APPARATUS:
Sodium thiosulfate, tincture of iodine (drug store), two drinking
glasses.
PROCEDURE:
Pour five drops of tincture of iodine into a dry glass and set aside
until the liquid dries. Pour water into the glass and note the wine
color. Dissolve five measures of sodium thiosulfate in a tumbler
half filled with water. Add this solution to the red solution. Note
how the wine color vanishes.
EXPERIMENT No. 889 Changing Water To
"Wine" And “Milk"
(CL-38, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, phenolphthalein solution, strontium chloride,
sodium bisulfate and three glasses.
PROCEDURE:
Dissolve three measures of sodium carbonate in a glass three
quarters filled with water. Place three drops of phenolphthalein
solution in the second glass. Put three measures of strontium
chloride and three measures of sodium bisulfate into the third
glass. Pour the first solution into the second, and the second into
the third, stirring vigorously for a while. Note how the solutions
change from a colorless to a red and then to a white liquid.
EXPERIMENT N0. 890 Changing Water To "Ink" And
“Milk”
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tannic acid, strontium chloride, ferric ammonium sulfate, two
glasses and a pitcher.
PROCEDURE:
Put two measures of tannic acid in one glass and three measures of
strontium chloride in the other, Add enough water to each glass to
dissolve the solids. Dissolve four measures of ferric ammonium
sulfate in the small pitcher together with a glass of water. Pour
this solution into each glass. Note the blue and cloudy colors in
the respective glasses.
EXPERIMENT No. 891 Transforming Water To "Milk"
(CL-33, CL-44. CL-55, CL-66, CL-77)
APPARATUS:
Manganese sulfate, sodium carbonate, two glasses.
PROCEDURE:
Dissolve three measures of manganese sulfate in a glass half filled
with water. Dissolve three measures of sodium carbonate in another
glass half filled with water. Pour one solution into the other. Note
the formation of a milk-like precipitate.
EXPERIMENT No. 892 A Pitcher Of Milk And Ink
(CL-38, CL-44, CL-55, CL-66. CL-77)
APPARATUS:
Pitcher, four glasses, manganese sulfate, strontium chloride, tannic
acid, ferric ammonium sulfate.
PROCEDURE:
Place the following chemicals in the glasses together
LIONEL
CHEM-LAB 315
with a few drops of water: Glass No. 1, three measures of manganese
sulfate. Glass No. 2, three measures of strontium chloride. Glass
No. 3, two measures of tannic acid. Glass No. 4, two measures of
ferric ammonium sulfate. Fill the pitcher half way with water. Pour
water into each of the glasses and then return the contents of
glasses No. 1 and No. 2 to the pitcher. Refill these two glasses
from the pitcher. Note the milky color in these glasses. Pour the
contents of glasses No. 3 and No. 4 into the pitcher and then back
into the glasses. Note the inky coloration. The first precipitate is
due to the formation of strontium sulfate. The ink appearance is due
to the presence of tannic acid and ferric ammonium sulfate.
EXPERIMENT No. 893 Mystery Solution
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, phenolphthalein solution, red litmus paper,
stirring rod and a glass.
PROCEDURE:
Dissolve two measures of sodium carbonate in a glass one quarter
filled with water. Add to this two drops of phenolphthalein
solution. Note the red color. Dip the litmus paper into the
glass. Note the change in color of the litmus paper in the red
solution.
EXPERIMENT No. 894 Making A Colorless Liquid
White
(CL-11, CL-22, CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Calcium oxide, sodium carbonate, sugar, two glasses, and glass
tubing.
PROCEDURE:
Dissolve two spoonfuls of sugar in a glass of water. Add to this two
measures of calcium oxide and stir for a minute or two. Set aside
until the precipitate settles at the bottom. Pour some of the clear
solution into another glass. Blow into this solution through the
glass tubing. Note how the liquid gradually becomes white.
EXPERIMENT No. 895 Changing Water To "Milk" And
To “Ink"
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Ferric ammonium sulfate, strontium chloride, tannic acid and three
glasses.
PROCEDURE:
Dissolve two measures of ferric ammonium sulfate in a glass three
quarters full of water. Place seven measures of strontium chloride
in the second glass adding enough drops of water to dissolve the
chemical. Add two measures of tannic acid to the third glass with
two or three drops of water. Pour the first solution into the second
and the second into the third. Note how the water changes to white
and then to black.
EXPERIMENT No. 896 Wine And Bluing From One
Pitcher
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Pitcher, four glasses, sodium carbonate, phenolphthalein solution,
ferric ammonium sulfate and sodium ferrocyanide.
316
CHEMICAL MAGIC
PROCEDURE:
Place the following chemicals in the glasses together with a few
drops of water: Glass No. 1, one measure of sodium carbonate. Glass
No. 2, two or three drops of phenolphthalein solution. Glass No. 3,
two measures of ferric ammonium sulfate. Glass No. 4, two measures
of sodium ferrocyanide. Fill the four glasses halfway with water
from the pitcher. Pour the contents of glasses No. 1 and No. 2 into
the pitcher and then back into the glasses again. Note the wine
color. Pour the contents of Glasses No. 3 and No. 4 into the pitcher
and then back into the glasses again. Note the blue coloration.
EXPERIMENT No. 897 Pouring Wine And Water From
The Same Pitcher
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Phenolphthalein solution, sodium carbonate, tartaric acid, two
glasses and a pitcher.
PROCEDURE:
Place a half measure of sodium carbonate in a glass and add a few
drops of water to dissolve it. Put one measure of tartaric acid into
another glass and add a few drops of water to dissolve it. Mix in
the pitcher a few drops of the phenolphthalein solution with a glass
of water. Pour some of this into each of the glasses. Note the red
color change in the glass containing the sodium carbonate.
Phenolphthalein in the presence of an alkali salt imparts a red
color to the solution.
EXPERIMENT No. 898 An Amazing Red Color From Blue
(CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Phenolphthalein solution, ferric chloride, sodium ferrocyanide,
sodium silicate solution, three glasses and a stirring rod.
PROCEDURE:
Pour three drops of phenolphthalein solution into a glass half
filled with water and stir. Place one third measure of ferric
chloride and one third measure of sodium ferrocyanide into another
glass half full of water and stir. Fill a third glass with a
spoonful of sodium silicate solution and two spoonfuls of water and
stir the solution. Pour the first and second simultaneously into the
third. Note the resulting bright red liquid.
EXPERIMENT No. 899 Changing Water To Different
Colors
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Tannic acid, sodium bisulfate, sodium salicylate, ferric ammonium
sulfate, four glasses and stirring rod.
PROCEDURE:
Fill each of the four glasses halfway with water. Dissolve the
following chemicals in the glasses by stirring. Glass No. 1, one
measure of tannic acid, Glass No. 2, five measures of sodium
bisulfate, Glass No. 3, one measure of sodium salicylate.
Glass No. 4, four measures of ferric ammonium sulfate. Pour a little
of the contents of glass No. 4 into each of the other three glasses.
Note the blue, yellow and red colors.
LIONEL
CHEM-LAB 317
EXPERIMENT No. 900 Transforming Red, White And
Blue
(CL-55, CL-66, CL-77)
APPARATUS:
Sodium ferrocyanide, phenolphthalein solution, calcium oxide,
aluminum sulfate, strontium chloride, ferrous ammonium sulfate,
ferric ammonium sulfate, tartaric acid and four glasses.
PROCEDURE:
Line up the four glasses in a row and place in them the following
chemicals: Glass No. 1, half measure of sodium ferrocyanide, four
drops of phenolphthalein solution and half measure of calcium oxide.
Glass No. 2, half measure of sodium ferrocyanide, three measures of
aluminum sulfate and three measures of strontium chloride. Glass No.
3, half measure of sodium ferrocyanide and half measure of ferrous
ammonium sulfate. Fill the glasses two thirds full of water and
dissolve the chemicals by stirring. The solution in the first glass
will be red, that in the second glass will be white and the third
blue. Dissolve two measures of ferric ammonium sulfate and two
measures of tartaric acid in a glass filled with water. Pour some of
this solution into the other glasses. Note the blue solution in all
three glasses.
EXPERIMENT No. 901 The Disappearing Red
(CL-33, CL-44, CL-55, CL-66, CL-77)
APPARATUS:
Sodium carbonate, phenolphthalein solution and glass tubing.
PROCEDURE: Dissolve one measure of sodium carbonate in a glass half
filled with water. Add to this two or three drops of phenolphthalein
solution. Note the red color. Blow into the liquid through the glass
tubing. Note that it becomes colorless.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook
